
Lung cancer with air lucency: a systematic review and clinical management guide
Highlight box
Key findings
• LCAL appears to encompass different entities with respect to demographics, risk factors, imaging appearance, and biologic behavior.
• Progression of a solid component of LCAL is often rapid and associated with nodal involvement and worse outcomes.
• Additional pulmonary sites of lung cancer are reported in ~1/3rd of cases (both synchronous and metachronous).
What is known and what is new?
• LCALs are poorly understood lesions, often not recognized until after marked cancer progression has occurred.
• We propose a categorization and a framework for management based on aggregated available evidence.
What is the implication, and what should change now?
• We suggest serial imaging surveillance of irregular thin-walled lesions with air lucencies; an initial phase of growth of the lucency is often slow but variable.
• We suggest the development of (or growth of) a solid component is a marker of progression that warrants intervention.
Introduction
Typically, non-small cell lung cancer (NSCLC) presents as a solid or subsolid ground glass (GG) nodule; a less known presentation is lung cancer with air lucency (LCAL). This knowledge gap has significant implications: 23% of missed or delayed diagnoses in a lung cancer screening trial involved an LCAL (1).
We undertook a systematic review of published literature on LCAL to address this issue, focusing specifically on the biologic behavior in order to develop a framework for clinical management.
Various terms have been used in association with LCAL, including lung cancers associated with cystic airspaces, cavities, bullous emphysema, and lung cancers with a “bubble-like” appearance and pseudocavitation. While lung cyst, cavity, pseudocavity, and bulla have specific formal definitions (2), these terms are often used loosely (interchangeably) in association with LCAL. Therefore, we included evidence related to any of these entities—using the term “lung cancer with air lucency” (i.e., LCAL) to refer to the entire spectrum of these lesions. We avoid the term airspace, which has a defined meaning in an imaging context (2). We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1199/rc).
Methods
A study panel was assembled consisting of early- through late-career Chest Radiologists, Pulmonologists, and Thoracic Surgeons without relevant conflicts of interest. Study questions were defined from a clinical practice perspective (Appendix 1). We conducted a systematic review and analysis according to PRISMA standards (3) (provided online). PubMed and EMBASE databases were searched using terms related to cystic, cavity, bulla, pseudocavity, and bubble-like, referring to pulmonary lesions (2000–2022, details in Appendix 2). Studies were included that provided information related to the questions, populations, outcomes, and criteria described in Appendix 1. Specific inclusion criteria for each table are listed in the legends. Because the available evidence consists entirely of case series, all are categorized as low-level evidence.
Due to the heterogeneity of the literature, a meta-analysis was deemed inappropriate. Evidence is presented along with relevant information about types of lesions, patients, and settings to facilitate accounting for differences and uncertainty when drawing overall conclusions.
Based on the review of available data on natural history, progression, interventions, and outcomes, we developed a clinical guide to patient management (details of the process in Appendix 2). The proposals seek to balance avoiding unnecessary intervention against consequential delays in addressing a lung cancer. The proposed protocol for observation, criteria for intervention, and approach to management required a consensus of all panelists.
Results
Description/characteristics
Definition of terms
In this review, we adhered as closely as possible to the Fleischner definitions of terms related to LCAL (2). The definition of a cyst is a lucency within normal lung parenchyma with a well-demarcated interface (of variable thickness, usually <2 mm); a cavity is a lucency within an area of pulmonary consolidation, mass, or nodule; a bulla is a focal lucency >1 cm sharply demarcated by a thin wall (≤1 mm), typically associated with adjacent emphysematous changes (2). Additionally, LCALs are sometimes described as having pseudocavitation or a bubble-like appearance. Pseudocavitation is defined as small (usually <1 cm) oval or round areas of low attenuation within a region of consolidation, mass, or nodule, representing spared parenchyma, normal or ectatic bronchi, or focal emphysema rather than cavitation (2). Bubble-like is not formally defined; it is often used when describing GG lesions but sometimes also solid lesions. In this paper, “bubble-like GG” specifically denotes small air lucencies within a GG lesion and pseudocavitation within a solid/consolidated region. Available evidence on bubble-like GG LCAL has been reported together with other cystic LCAL.
Figures S1-S4 provides representative computed tomography (CT) images of LCAL types. Cystic LCAL can be thin-walled (0–4 mm), have a GG component, focal wall thickening or nodularity, be circumferentially thick-walled (>4–15 mm), or become mostly or completely solid. Cavitary LCAL have thick irregular walls, presumably representing a mass with central necrosis. Bullous LCAL are contiguous with emphysematous bullae. Pseudocavitary LCAL involves a solid/consolidated lesion as opposed to bubble-like GG LCAL. Thus, the extent of the solid component of LCAL is varied.
Application to published literature
Most reports use terms loosely and include a mixture of types of LCAL. Seeking to achieve a uniform usage of terms and facilitate comparisons, we assessed the tumor descriptions, extent of solid components, and range of lesions included in published reports (details in Appendix 3). We categorized studies by predominant LCAL type, applying terms as formally defined as well as possible to published studies. There is a progression in the proportion of smoke-exposed individuals and the proportion of squamous carcinomas and other histotypes among studies predominantly focused on cystic, cavitary and bulla-associated lung cancers—suggesting these are not simply different presentations or states of progression of a single entity. To facilitate interpretation of the aggregated evidence, the categorization by predominant LCAL type and solid tumor extent of individual studies is included in the tables.
Incidence
The reported incidence of LCAL ranges from 1–18% (Table S1) (4-19). Studies primarily involved surgical patients; all cases were histologically proven lung cancer. The incidence is ~1–4% in studies involving predominantly cystic LCAL (4-8) vs. ~5–15% in studies involving predominantly cavitary, pseudocavitary, or bullous LCAL (9-19). In studies reporting a high incidence (>10%), the number of comparator cases seems low for unclear reasons (given volume characteristics of those institutions). No regional or temporal patterns in incidence are apparent.
Patient and tumor characteristics
The lobar distribution of LCAL mirrors the proportional size of the lobes (Figure S5). The distribution is similar among studies involving predominantly cystic vs. cavitary LCAL; data on pseudocavitary or bullous LCAL is limited (15). Approximately 66% of cystic LCAL are in the outer 1/3rd of the lungs (4,20,21).
The reported median age is 52–71 years (Appendix 3, Table A), but the age range is broad and includes patients in their 20s and 30s. The sex distribution varies widely (Figure 1) (4-14,17-29). The proportion of never-smokers also varies widely, partially reflecting the general smoking prevalence in the study regions (Figure 1, Appendix 3 Table A). Studies involving predominantly bullous LCALs report a markedly higher proportion of men and smoking exposure.
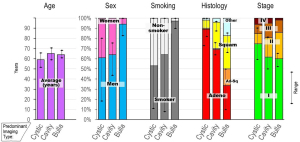
Patients and tumor characteristics grouped by predominant type of LCAL. Insufficient data is available for pseudocavitary LCAL. Results are depicted as an average (and maximum and minimum of individual studies) among the studies included in Appendix 3 Table A.
Adeno, adenocarcinoma; Ad-Sq, adenosquamous carcinoma; LCAL, lung cancer with air lucency; Squam, squamous carcinoma.
References (4-14,17-29).
Cystic LCAL are mostly (~90%) adenocarcinomas; the proportion decreases markedly among studies involving predominantly cavitary and bullous LCAL (Figure 1, Appendix 3 Table A). A wide range of histotypes are reported sporadically [e.g., carcinoid (6,14), small cell (14,17,18), pleomorphic carcinoma (12), sarcomatoid (21, 29), lymphoma (20,25), lymphangioma (30)]. Studies almost exclusively involve resected patients. As expected, most cases are stage pI (Figure 1, Appendix 3 Table A); this is less pronounced among studies involving predominantly cavitary or bullous LCAL (which involve tumors with a larger solid component and broad inclusion criteria). The rate of node involvement increases as the solid component of the primary tumor increases (5,12). However, whether pIII–IV cases are categorized as such because of additional foci in other lobes or nodal and distant metastases is unclear.
A high rate (~30%) of prior or synchronous separate lung cancers is noted among studies addressing this issue (8,27,28). Furthermore, additional cysts or GG nodules are reported in ~50% of patients (8,27,28).
Etiology
Limited and conflicting data leave it unclear whether LCAL is merely an unusual presentation of “regular” NSCLC or a distinct entity. Many studies comparing LCAL and contemporary surgical non-LCAL patients note differences with respect to sex, smoke exposure, and stage, but some show no differences (Table S2, Figure S6). There is little difference with respect to average age. These observations apply primarily to cavitary- or bulla-associated LCALs; data is limited for cystic LCAL. Data on genetic characteristics is too limited to make an assessment. Data on adenocarcinoma subtypes is conflicting: one study (13) found no difference in LCAL vs. non-LCAL tumors while another found statistically significant differences (10).
Speculative mechanisms regarding how the air lucency of an LCAL arises include central necrosis (perhaps due to insufficient blood supply), a check-valve phenomenon (airway obstruction leading to air entrapment), and cancer developing adjacent to a pre-existing bulla (perhaps related to chronic inflammation). There is some evidence consistent with each of these hypotheses in various studies (8,20,22,23,25,31). The data is vague and circumstantial; more than one mechanism may exist (8). However, it is unclear how defining a pathophysiologic mechanism underlying LCAL affects management.
Natural history
Cystic LCAL
A pattern of progression is emerging for cystic LCAL. When a precursor is seen, it is frequently a GG nodule, transforming over a median of 16 months into a thin-walled cyst, with or without a surrounding GG component (5,8,14).
The next phase of change is enlargement of the cystic air lucency without thickening of the wall in the study by Jung et al. (5). Typically, this occurs slowly (doubling in diameter over 3–10 years). But in ~1/3rd of cases, doubling occurs over 1–2 years, and in another ~20%, such rapid growth is observed after many years of stability. Development of a solid component without prior growth of the cystic air lucency occurs rarely (5). Other studies loosely corroborate this pattern of early progression (6). After development of a solid component, the size of the cystic air lucency generally decreases (Figure 2) (5).
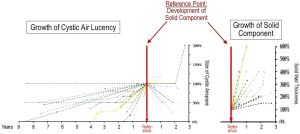
Patterns and rates of progression of air-filled and solid components. The reference point is the appearance of a solid component in a longitudinal assessment of patients with LCAL.
LCAL, lung cancer with air lucency.
Reproduced with permission from Jung et al., Ann Surg Onc 2020 (5).
The most significant change appears to be development of a solid region (i.e., a nodule or wall thickening). Once a solid component has appeared, progression occurs at a variable but often rapid rate (Figure 2). Jung et al. (5) observed doubling of the solid component thickness by 3 years in almost all cases; size doubling occurred in <12 months in ~2/3rd of cases. Fintelmann et al. (8) noted a volume doubling time (VDT) of ~250 days in progressing lesions (how VDT was measured is unclear).
The most ominous phase of progression is circumferential and/or more substantial wall thickening, or transformation into a completely solid nodule (Figure 3) (5,12). The time course of this phase of change is unclear. Other studies loosely corroborate this pattern of progression (4,28).
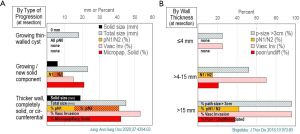
In 2 studies reporting this information, the incidence of negative prognostic factors increased markedly with development of an increasing solid component (A) by type of progression and (B) by solid size at time of resection. The horizontal axis represents mm of size or percent incidence of the factor.
Micropap, micropapillary adenocarcinoma subtype; path, pathologic; poor/undiff, poorly or undifferentiated carcinoma; p-size >3 cm, percent of patients with pathologic tumor size >3 cm; Solid, solid adenocarcinoma subtype; Vasc Inv, vascular invasion.
Data taken from Jung et al., Ann Surg Onc 2020 (5) and Shigefuku et al., J Thor Dis 2018;10:973-83 (12).
Most studies of cystic lucencies that developed a solid component have reported further progression in the vast majority (especially if observed ≥12 months) (4-6,28). However, most reports included only histologically confirmed (i.e., resected) cancers, thus excluding cases that were not suspicious and remained so. Some studies noted stability in ~20–30% of cystic lesions (including some with nodularity or wall thickening), but the duration of observation was not reported) (8,28). Thus, whether circumstances exist that ensure ongoing stability is unclear.
Multiloculated or bubble-like GG LCAL
There is insufficient data to define the natural history of multiloculated cystic or bubble-like GG LCAL. One study (8) notes that multiloculated cysts most often developed from a uniloculated cyst; rarely, multiloculated cysts become single cystic spaces. Jung et al. (5) vaguely imply that multiloculated lesions are a late manifestation of progression.
Cavitary, bullous, pseudocavitary LCAL
Limited data is available on progression of cavitary LCAL; over a mean of 16 months 87% progressed, including 24% that became completely solid (28). A volume doubling time of ~3.5 years was reported among 9 patients with what appears to be mostly pseudocavitary LCAL (32). No data is available on the progression of bullous LCAL.
Stage progression during observation
Little data is available regarding stage progression during observation. Anecdotally, both development of mediastinal node involvement and lack thereof has been described during 1–2 years of observation of cystic LCAL (23). The incidence of node involvement increases as the solid size increases (0, 15%, and 42% for ≤4, 4–15, and >15 mm wall thickness, respectively, in predominantly cavitary LCAL) (12). Comparing across studies, the incidence of stage II–IV is higher in studies involving more extensive tumors (e.g., predominantly cystic vs. cavitary or bullous LCAL; see Figure S7, Appendix 3 Table A).
Clinical management
Differentiating benign vs. malignant lesions
There is a substantial overlap in the CT appearance between benign and malignant lesions with air lucencies (33), no single radiographic feature reliably differentiates these. A 1980 analysis of chest radiographs (CXR) suggested a wall thickness of >4 mm was a marker of malignancy (34). Recent studies involving CT scans report conflicting results regarding wall thickness as a differentiator (31,35,36).
Considering clinical aspects together with radiographic features is more clinically relevant (e.g., signs/symptoms of infection, immunocompromised state, autoimmune disease, endemic exposures) (33). Acutely ill patients or those with one (or more) rapidly progressive lesions with air lucencies warrant an infectious and/or inflammatory work-up (and are outside the scope of this paper). Our analysis addresses patients in a stable state of health noted to have an air lucency. The clinical presentation generally allows initial triage towards further testing or surveillance in most patients; a correct clinical diagnosis is made in ~80% by experienced radiologists and clinicians (37). Observation for progression vs. stability (or regression) is arguably the best differentiator between benign and malignant lesions with air lucencies.
A mental construct of the appearance and evolution of various lesions with air lucency is shown in Figure S8, based on well-established observations (e.g., general stability of simple cysts, pattern of progression of cystic LCAL, and subacute/chronic inflammatory processes) and presumptions (e.g., cavitary LCAL arising from central necrosis of a solid lesion, lung cancer arising adjacent to preexisting bullae appearing as a bullous LCAL). This schematic suggests that earlier detection and observation of changes may allow identification based primarily on imaging. Late manifestations are difficult to differentiate by imaging (but generally warrant definitive diagnosis—i.e., invasive biopsy).
Diagnostic tests
18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) has limited utility in early diagnosis of LCAL, but most LCAL with a substantial solid component are metabolically active. In one study predominantly involving fairly extensive cavitary lesions, 67% (14/21) showed moderate/marked FDG uptake; in most initially FDG-negative lesions moderate/marked uptake ensured over 12–24 months as the lesion progressed (28). Moderate/marked FDG uptake was confirmed in another study involving thick-walled cavitary LCAL (38). However, in a study involving predominantly thin-walled cystic tumors, 83% (5/6) had no FDG uptake (24). A study of predominantly pseudocavitary LCAL reported generally low FDG uptake (15). Studies with a broad range of the extent of the solid component report varied results (27).
To assess the efficacy of invasive diagnostic procedures, we defined 3 outcomes as most clinically relevant. First, a result was considered helpful if it clarified appropriate further management (treatment of malignancy, specific antibiotics, or that no intervention was necessary); non-diagnostic or nonspecific results were considered unhelpful. Second, sensitivity was defined as the rate of a correct diagnosis leading to a specific treatment among cases with an infection or malignancy. Lastly, we tracked the rate of missing a malignancy or an infection (among all non-diagnostic and non-specific results).
Reports of CT-guided biopsies (Table 1) involve both thin-walled cystic lesions and thicker cavities, and include core biopsy, needle aspiration, and needle washing (cytology/microbiology of saline used to wash a cavity) (15,39-44). Biopsies of cystic/cavitary lesions are reasonably safe (chest tubes inserted in 0–9%, pneumothorax in 6–27%); using a smaller needle (22-gauge) may lower complications slightly. Biopsy results were helpful in ~75% of cases in larger studies. The sensitivity for diagnosing cancer or a specific infection was high (~90%). However, a substantial false negative rate (~10–30%) for cancer or infection remains among non-diagnostic and non-specific cases. It is not clear that larger biopsies have a higher yield; one study reported that core biopsy yielded no additional information over needle aspiration (median wall thickness 12 mm) (42). Conflicting results are reported whether yield is associated with wall thickness (39,42,43).
Table 1
Ordered by predominant lesion type, biopsy technique
| 1st Author, reference |
n | Technique | Needle gauge | Predominant type | Solid size (mm) | % Pneumothorax | % Chest tube | % Helpfula | % Sensitivityb Cancer/Infection | % FN for Cancerc | % FN for Infectionc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shin (39) | 32 | Core | 18–20 | Cyst | ≤4 | 27 | 3 | 69 | 88 | 25 | 13 |
| Nakahara (40) | 26 | Wash | 22 | Cyst | ≤4 | 7 | 0 | 77 | 90 | 0 | 29 |
| Belet (41) | 16 | Wash | 22 | Cyst | ≤5 | 6 | 0 | 44 | 88 | 11 | 0 |
| Kiranantawat (42) | 53 | Asp d | 19 | Cav | Ext | 25 | 9 | 81 | 88 | 30 | 30 |
| Zhuong (43) | 102 | Asp | 18–20 | Cav | Ext | 9 | – | 79 | 89 | 29 | 19 |
| Black (44) | 12 | Wash | 22 | Cav | – | 8 | 0 | 83 | 100 | (50)e | (0)e |
| Kojima (15) | 21 | Bronch | – | PsCav | Ext | – | – | 33 | 33 | – | – |
Inclusion criteria: studies reporting diagnostic reliability in ≥10 cases of lesions with air lucency 2000–2022.
a, considered helpful if result clarified appropriate further management (treatment of malignancy, specific antibiotics, or that no intervention was necessary); b, sensitivity refers to the rate of a definitive diagnosis (specific benign or cancer diagnosis) among all cases; c, among non-diagnostic and non-specific cases (includes “suspicious” cases); d, additional Core needle biopsy in 34%; e, <5 patients (in parentheses because calculating a percentage is questionable).
Asp, percutaneous needle aspirate; Bronch, bronchoscopy; Cav, cavitary; Core, core needle biopsy; Ext, extensive; FN, false negative rate; PsCav, pseudocavitary; Wash, percutaneous needle puncture/washing of the air cyst.
Observation protocol
A proposed observation protocol is shown in Table 2, based on available data on natural history, progression, and outcomes. The protocol seeks to balance avoiding procedures for benign lesions and timely intervention when malignancy is suggested.
Table 2
| Clinical scenario | Observation protocola,b | Justification |
|---|---|---|
| Simple cyst—round, paper-thin wall, within normal lung parenchyma | None needed | Common benign finding |
| Thin-walled cystic air lucency, irregular shape (not round or oval) | LDCT q1 yr ×5; if increasing air cyst size → continue LDCT q1 yr till 10 yrs (total) |
Low suspicion, likely benign, but some LCAL initially progress slowly |
| Thin-walled irregular cystic air lucency… evolving from a GG lesion or with surrounding GG; or with non-uniform wall or bubble-like GG/multiloculated cyst |
LDCT q6 mo. ×2; if no change → continue LDCT q1 yr till 10 yrs (total) |
Suspicious lesion; rapid progression often ensues |
| Thin-walled irregular cystic air lucency with small solid component (<6 mm on LW or <2 mm on MW) | LDCT q3 mo. ×2; then in 6 mo., then q6–12 months for 1 yrif no change over 2 yrs → continue LDCT q1 yr till 10 yrs (total) | Highly suspicious lesion |
| Thick-walled cavitary lesion and clinical setting consistent with infectious or inflammatory process | Short interval LDCT (~6–8 weeks) | Possible resolution over time |
| Bullous emphysema with no or <4 mm nodule (LW) | None needed; optional LDCT in 1 yr if a <4 mm nodule existsc | Common benign finding |
| Regional bulla/emphysema with adjoining 4–6 mm solid nodule (LW) | LDCT q1 yr ×2c | Probably benign but cautious approach given limited data in setting of adjoining bulla |
| Regional bulla/emphysema with adjoining 6–8 mm solid nodule (LW) | LDCT q6 mo. ×2, then in 1 yrc | Suspicious lesion; surveillance similar to high-risk lesion by Fleischner Society |
| Pseudocavitation (<1 cm lucencies in solid/consolidated region (usually ~2 cm) | PET, if no/low activity → LDCT in 3–6 mo. then q6–12 mo. till 2 yrs (total)c |
Moderately suspicious but generally large and solid enough for reliable PET assessment |
GG, ground glass; GGN, ground glass nodule (pure GG, heterogenous on LW, or part-solid on MW); LCAL, lung cancer with air lucency; LDCT, low-dose computed tomography (non-contrast); LW, lung window setting; mo, months; MW, mediastinal window setting; PET, 18F-fluoro-2-deoxy-D-glucose positron emission tomography; yr, year.
a, CT scans should be done with thin slice thickness (≤1 mm); b, ongoing annual surveillance may be warranted in patients meeting criteria for LDCT screening for lung cancer; c, further annual surveillance may be warranted if suspicion of a slowly progressing lung cancer remains.
Benign thin-walled pulmonary cysts are common, increasing slightly with age (5% age 40–50 years, rising to 13% age ≥80 years) in a longitudinal population cohort study (45). These are mostly solitary, peripheral, round or oval cysts in the lower lobes, and not associated with smoking or emphysema. Most cysts remained stable (median interval 6 years), but increased in size (>2 mm) in 36% and rarely decreased (45). No progression to cancer was reported in this cohort (45).
A prolonged course of regular observation of irregular cystic lesions is warranted. The natural history data of cystic LCAL suggests that progression is often rapid once a solid component appears. The observation that in a screening context, more LCAL are seen in annual repeat rounds than at baseline also suggests an aggressive nature (6). A substantial solid component is associated with markedly worse survival (see subsequent Outcomes section). Therefore, an observation protocol should be sufficiently intensive to allow early intervention. We suggest bubble-like GG lesions be observed in a manner similar to thin-walled cystic air lucencies with a non-uniform wall.
The approach to a (thick-walled) cavitary lesion depends on the context and presumptive diagnosis. If the patient has signs/symptoms of vasculitis or infection or is immunosuppressed, regression with appropriate treatment is likely. Persistence despite antibiotic/anti-inflammatory treatment generally warrants further diagnostic work-up. Malignancy should be suspected in a cavity occurring in the absence of infection or a systemic disease associated with pulmonary lesions.
A typical bulla—a thin-walled air lucency with surrounding emphysema—warrants no imaging follow-up. However, if such a bulla has a significant adjoining solid nodule (>4–8 mm on lung windows), we propose surveillance similar to the Fleischner high-risk protocol (46).
There is little information regarding the behavior of pseudocavitary (solid) lesions on which to base a surveillance protocol. In the absence of prior imaging, we suggest further investigation (e.g., FDG-PET) upon initial detection of a pseudocavitary lesion. If residual scar is suspected (low FDG uptake or stability), we propose ongoing surveillance for ≥2 years, with an initial interval of 3–6 months depending on the level of confidence (e.g., lack of FDG uptake is less reliable in <2 cm lesions).
Triggers for diagnostic evaluation and clinical management
Cystic lesions
We propose that for a cystic air lucency, intervention is warranted upon appearance of a solid component (Figure 4, Table 3). This is based on several arguments: (I) the solid component growth rate is often rapid (although variable), (II) node involvement increases substantially with development and progression of the solid component, and (III) survival decreases with progression of the solid component. The evidence for this comes primarily from 2 retrospective studies (5,12), which parsed oncologic features and survival by patterns of change of predominantly cystic LCAL (Figure 3). Other studies involving both cystic and cavitary LCAL provide indirect evidence supporting intervention before progression of a solid component (4,47).
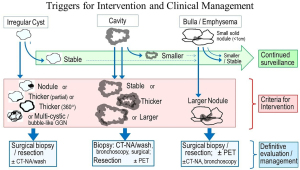
CT-NA, CT guided needle aspiration or biopsy, possibly including wash of the air-filled space; GGN, ground glass nodule; PET, 18F-fluoro-2-deoxy-D-glucose positron emission tomography.
Table 3
| Clinical scenario | Trigger | Justification |
|---|---|---|
| Thin-walled irregular cystic lucency | New solid area (≥6 mm on LW or ≥2 mm on MW) | High likelihood of lung cancer, outcomes good if treated when only small solid component |
| Thin-walled irregular cystic lucency with small solid component (<6 mm on LW, <2 mm on MW) | Progression of solid component (↑ nodule size, wall thickness, or portion of circumference by ≥6 mm on LW or ≥2 mm on MW) | High likelihood of lung cancer, outcomes good if treated when only small solid component |
| Cavitary lesion (without clinical signs of acute infection or systemic inflammatory disease) | Persistence after 1–2 months | High likelihood of lung cancer or inadequately treated benign etiology |
| Progression after 1–2 months; Upon initial detection if clinical likelihood of infection is low and with risk factors for lung cancer |
High likelihood of lung cancer and concerning outcomes; presumably worsened by further delay | |
| Pseudocavitary appearance in a solid or consolidated area | Upon initial detection | Limited data; generally larger size suggests more investigation is justified |
| Regional bulla / emphysema with adjoining 4–8 mm solid nodule (LW) | Progression of the solid nodule | High suspicion for lung cancer; outcomes presumably worsened by further delay |
| Regional bulla / emphysema with adjoining larger solid nodule (>8 mm LW) | Upon initial detection | High suspicion for lung cancer; outcomes presumably worsened by further delay |
LW, lung window setting; MW, mediastinal window setting.
Would intervention once growth of a thin-walled cyst (without a solid component) occurs be even better? Arguments against this include: (I) clear criteria aren’t available differentiating benign from malignant cysts, (II) often growth of the air lucency of a cystic LCAL is very protracted, and (III) the risk of a potential malignancy must be balanced against the risk of an intervention and other potential serious conditions.
We propose the following definition of a solid component as an indication for intervention: ≥6 mm on lung windows (or ≥2 mm on mediastinal windows) by greatest dimension on a thin slice (~1 mm) CT. In the absence of a focal nodule, the maximal wall thickness should be measured. This mirrors what has been proposed for GG nodules (48). We speculate that the greatest dimension of a solid component may be the most useful criterion for progression of a cystic LCAL (vs. degrees of partial circumference thickening or progression of a surrounding GG component). Judging by images of LCAL in published series, intervention at the point of the proposed criteria would be much earlier than in most reported cases.
We propose that a surgical biopsy is generally best once progression consistent with a cystic LCAL is observed (Figure 4, Table S3). In such LCAL, FDG-PET is not useful (little avidity), and needle aspiration is unlikely to alter the need for resection (a specific benign diagnosis is unlikely and a non-specific result has a substantial false-negative rate).
Cavitary lesions
Cavitary lesions have a substantial solid component by definition. When the clinical setting makes lung cancer more likely than infection or vasculitis (e.g., lung cancer risk factors, lack of signs/symptoms of infection, or predisposing conditions), we propose pursuing a diagnostic evaluation upon initial detection (Table 3). If the clinical setting makes infection or vasculitis more likely, re-imaging after a short interval is reasonable; intervention is warranted if the lesion persists and certainly if it progresses (even if it is benign). These proposals are based on a high clinical concern of malignancy and generally poor outcomes (47)—presumably worse with further delay.
If the suspicion of a cavitary malignancy is high, we propose a surgical approach to diagnosis is justified; if an inflammatory cause is likely, a CT-guided biopsy is recommended (Figure 4, Table S3). However, a non-specific needle biopsy result must be pursued further due to a substantial false-negative rate. FDG-PET has little utility for diagnosis (likely positive in benign and malignant cases); however, FDG-PET may be useful as a staging evaluation of cavitary LCAL.
Bullous lesions
A larger nodule in a region of bullous emphysema in a patient with risk factors for lung cancer would generally justify a diagnostic evaluation at initial detection. FDG-PET may be helpful in larger lesions to differentiate scar vs. malignancy or infection, but a negative FDG-PET requires further surveillance. We propose serial CT imaging for a small nodule, given challenges in performing a needle biopsy and the false-negative rate of FDG-PET; if the lesion progresses, a definitive diagnosis becomes necessary (Table 3).
We suggest a definitive surgical biopsy is best for larger or progressing nodules with adjoining bullae or regional emphysema (i.e., high suspicion of cancer), whereas those concerning for infection should undergo a needle or bronchoscopic biopsy (Figure 4, Table S3). In the latter scenario, a non-specific biopsy result will generally demand a definitive surgical biopsy. FDG-PET is unlikely to avert the need for definitive histologic/microbiologic diagnosis in progressing lesions, but may be useful as a staging evaluation when malignancy is suspected.
Pseudocavitary lesions
We propose that a pseudocavitary appearance in a solid or consolidated area be considered suspicious enough to warrant investigation. We suggest FDG-PET as a first step with the rationale that FDG-PET may be useful in differentiating malignancy from scar, given the generally larger size of such lesions. Serial imaging for several years is warranted if FDG-PET is negative; if there is FDG uptake, a tissue diagnosis should be pursued.
Definitive treatment recommendations
Most reported LCAL have been managed by resection (lobectomy > segmentectomy > wedge), only sporadically by ablative techniques or systemic therapy. Direct data comparing treatments was not identified—thus recommendations are based on extrapolation from potentially relevant similar tumors and rationale. Reasonable speculation is that data from the recent Japan Clinical Oncology Group study (JCOG0802) (49) (a randomized controlled trial of segmentectomy vs. lobectomy for ≤2 cm tumors that are partially GG up to completely consolidated) may apply to a LCAL with a small solid component; The JCOG0802 study reported similar overall survival (OS) and recurrence-free survival (RFS) for lobectomy and segmentectomy. This is balanced against the general data for solid cI NSCLC that suggests survival is incrementally worse after lobectomy vs. segmentectomy vs. wedge vs. ablation (50,51). The high frequency of additional lung cancers suggests a need to balance management of the LCAL at hand with the ability to address future cancers.
We propose that segmentectomy may be best (if anatomically suitable) if intervention is undertaken at the first appearance of a solid component (based on infrequent node involvement and a propensity for additional lesions). We propose a pathologic margin of >1 cm be sought (extrapolating from traditional lung cancers) (50). For LCAL with more substantial solid components, we suggest a lobectomy is best (based on the high rate of node involvement and poor survival) (5,8). Regardless of the type of resection, we suggest a systematic node sampling or dissection be performed.
We propose that lobectomy may be best for predominantly cavitary, pseudocavitary, or bullous LCAL. This is based on the generally larger solid size of these tumors, generally worse outcomes, no recognized propensity to develop additional pulmonary foci, and lobectomy being the traditional standard for early-stage NSCLC.
Very limited data is available regarding response to systemic therapy. One study noted a >50% response to neoadjuvant therapy in 6 of 6 patients (without reporting what was given) (52). A response to immunotherapy (± chemotherapy) was observed in 2 of 3 patients (53). A response to tyrosine kinase inhibitors has also been reported (7,24). Assessment of response is difficult in these lesions, in which the air lucency component complicates the usual measurements. A method of response assessment has been proposed, based on either the area or volume of the solid component (52).
Long-term outcomes
Several studies report that long-term outcomes are markedly different for resected cystic LCAL with thin walls vs. a growing solid component and especially vs. thick-walled or mostly solid lesions (Figure 5) (5,12). This is consistent with the dramatically increasing rate of node involvement along this progression towards more substantial solid components (Figure 3). Other studies corroborate this (54).
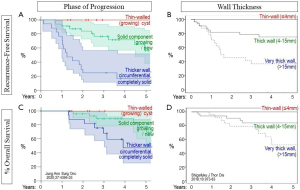
Outcomes by the type of progression (A,C) and by wall thickness (B,D) at time of resection. Top row depicts recurrence-free survival (A,B); bottom row depicts overall survival (C,D).
Reproduced with permission from Jung, Ann Surg Onc 2020 for (A,C) (5) and Shigefuku, J Thor Dis 2018;10:973-83 for (B,D) (12).
Long-term outcomes by predominant type of LCAL or stage are not well-defined; data is limited and based on various historical editions of stage classification. A study involving predominantly cystic LCAL found a 5-year RFS of 87% (86% underwent lobectomy, 92% were N0) (54). Studies involving predominantly cavitary LCAL report 5-year OS of ~40–70% for all stages (~80% for pI) (9,10,12,13,21). In studies with predominantly bullous LCAL, 5-year OS is ~50–90% (all stages) and ~65–85% for pI, ~40% for pII (17-19). The resection extent was unclear in these studies but presumably primarily involved lobectomy. One study involving pseudocavitary LCAL reported surprisingly good results: 5-year OS of 100% and RFS of 95% (26 patients, 92% underwent lobectomy, average tumor size 35 mm) (15).
Several studies compare survival after resection of cystic/cavitary cancers to NSCLC in general (all stages combined), with some noting worse survival for LCAL (9,10), some the opposite (17,54), others no difference (18), or results varying by stage (19). How stage was defined in the latter study is unclear, given the common occurrence of additional lesions and uncertainty how size is measured. The current staging system counts only the solid or invasive tumor (55) but how to report this for LCAL is undefined.
The pattern of recurrence is unclear. Several studies (involving predominantly cavitary LCAL) report more frequent locoregional than distant recurrences (9, 10); whether this involves regional nodes or additional pulmonary sites is unclear. Case reports note diffuse bilateral lung involvement without distant metastases (56-58); others document extrathoracic metastases (24,59), sometimes even with primary sites with only a limited solid component (23,24).
Prognostic factors are not well-defined. Two studies involving predominantly cavitary LCAL reported that wall thickness was prognostic for OS in multivariate analysis (12,47) (in addition to node status in one) (47). One study noted a univariate association of RFS with total size (solid plus air components) among thicker or nodular cystic LCAL (54).
Discussion
The incidence of LCAL is not insignificant, yet they are not well understood. LCAL are often recognized in retrospect when substantial progression has occurred and outcomes are disappointing. Improved understanding of the biologic behavior of LCAL and early recognition are needed. This prompted us to undertake a comprehensive review and develop a framework for clinical management. Key conclusions are summarized in the Highlight box.
Providing a clear summary of the topic is difficult because the available evidence is limited and muddled. It appears that LCAL encompasses a mixture of entities in many reports—probably because of similar imaging features in later stages of progression. We propose considering cystic, cavitary, and bullous LCAL as potentially distinct entities, but acknowledge this is based on an intuitive impression of available studies. Whether tumors with small (<1 cm) air lucencies in solid/consolidated lesions (pseudocavitary LCAL) are part of this spectrum is unclear. Small air lucencies in a GG nodule (bubble-like GG) seem to be part of cystic LCAL.
The finding that different authors mean different things by terms associated with LCAL seriously undermines our ability to communicate about the topic. In our review, we have adhered as much as possible to the Fleischner definitions (2). We consider particularly important features of the terms to be: cystic—irregular shape, and (at least initially) relatively thin-walled; cavitary—irregular thick-walled; bullous—within a region of emphysematous lung parenchyma; pseudocavitary—small (<1 cm) air lucencies within a dense mass, nodule or consolidation; bubble-like—small air lucencies within a GG lesion. We urge future authors to adhere to a standard definition such as Fleischner, or at least to clearly define what they mean by the terms used.
We think it is premature to attempt to articulate a definition of separate entities. Further assessment by the broader community is needed to corroborate or refute this mental framework. If a consensus emerges that the framework has value, clinically applicable definitions can be developed.
Imaging features are only one characteristic to consider. The histotype may be a defining feature; one study noted differences between squamous and adenocarcinomas despite all being thick-walled cavities (11). Nevertheless, outliers exist (thin-walled squamous LCAL or thick-walled adenocarcinomas) (10,17,20,24-28). Genetic features are relatively unexplored. However, the key is identification of markers that predict tumor behavior—i.e., when to intervene and how to treat these patients. Tabulating occurrence of particular features is less helpful than linking profiles to patterns of progression and long-term outcomes.
The relationship between cystic LCAL and ground glass/lepidic (GG/L) adenocarcinomas is unclear. Indeed, many cystic LCAL have a GG component. However, GG/L lung cancers generally exhibit indolent behavior and excellent outcomes (even when part-solid), whereas cystic LCAL are generally reported to be aggressive with poor outcomes when a solid component has appeared. Is this merely a matter of widespread recognition of GG/L tumors and early management vs. limited awareness of LCAL and late intervention? The reported high incidence of more aggressive adenocarcinoma subtypes among more advanced cystic adenocarcinomas suggests GG/L and cystic lung cancers are different.
How to interpret the frequent occurrence of additional lung cancers in patients with an LCAL is unclear. Multifocal adenocarcinoma is a recognized entity; these are GG/L tumors with a markedly diminished propensity for nodal and distant metastases despite frequent additional pulmonary sites of disease (60). These lesions are not viewed as a manifestation of disseminated disease (and additional foci in the lungs are classified as T(m) and not as T3, T4, or M1a) (60). Stage classification of LCAL with respect to additional pulmonary tumors has not been defined. The frequency of nodal and distant metastases among LCAL suggests they should be considered distinct from multifocal adenocarcinomas.
The updated Lung CT screening Reporting & Data System (LungRADS) classification (November 2022, version 2.0) for CT findings during lung cancer screening includes for the first time lesions with air lucency (but doesn’t include discussion of the data or rationale underlying the classification and recommendations) (61). LungRADS classifies an “atypical lung cyst” as category 3 (probably benign, recommend CT in 6 months) if thick walled with a growing cystic component, category 4A (suspicious, recommend CT in 3 months) if thick-walled or multiloculated and category 4B (very suspicious, recommend clinical/diagnostic evaluation) if there is increasing thickness of a thick-walled cyst or a multiloculated cyst that is either growing, becoming increasingly loculated or developing a GG, consolidated or nodular component. Thus, the wording of LungRADS primarily addresses thick-walled and multiloculated cysts, and associates greater concern with increasing overall size, loculation, thickness of thick-walled lesions, and the development of new GG or denser components.
Our review and recommendations were developed independently, prior to the release of LungRADS version 2.0. Our recommendations are designed for a non-screening context (i.e., incidentally discovered lesions in patients regardless of smoking history and outside of an annual surveillance setting), and focused on clinical management (whereas LungRADS is primarily intended to codify reporting of imaging studies). We separately proposed a protocol for observation, triggers for intervention and specific interventions. Furthermore, we parsed these recommendations by changes in density, nodule size and wall thickness to a greater degree than LungRADS. We concluded that the size of the air lucency component is not particularly useful (other than to suggest continued surveillance if growing), and focused on the development or growth of a solid or consolidated component, even if associated with a unilocular, thin-walled lesion with air lucency. We may have focused too little on multiloculated air lucencies (mostly because we could identify little published data defining the risk and outcomes associated with such lesions). Assessment of how well our recommendations as well as those of the LungRADS panel function in clinical application will surely lead to further refinements.
Conclusions
We hope this effort to collate and logically assemble the available evidence provides a footing for progress and facilitates further research. We expect this will reveal flaws in our interpretation and way of thinking about these tumors; we welcome this as a sign of more robust progress. We hope this systematic review will stimulate interest and lead to a better understanding in the future. For the present, however, we aim to raise awareness, promote earlier recognition, improve timing of interventions, and achieve better outcomes for these patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1199/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1199/coif). EMM reports honorarium for lectures from Bristol-Myers Squibb, Boehringer Ingelheim, and AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scholten ET, Horeweg N, de Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol 2015;25:81-8. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [Crossref] [PubMed]
- Shen Y, Xu X, Zhang Y, et al. Lung cancers associated with cystic airspaces: CT features and pathologic correlation. Lung Cancer 2019;135:110-5. [Crossref] [PubMed]
- Jung W, Cho S, Yum S, et al. Stepwise Disease Progression Model of Subsolid Lung Adenocarcinoma with Cystic Airspaces. Ann Surg Oncol 2020;27:4394-403. [Crossref] [PubMed]
- Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Guo J, Liang C, Sun Y, et al. Lung cancer presenting as thin-walled cysts: An analysis of 15 cases and review of literature. Asia Pac J Clin Oncol 2016;12:e105-12. [Crossref] [PubMed]
- Fintelmann FJ, Brinkmann JK, Jeck WR, et al. Lung Cancers Associated With Cystic Airspaces: Natural History, Pathologic Correlation, and Mutational Analysis. J Thorac Imaging 2017;32:176-88. [Crossref] [PubMed]
- Kimura H, Saji H, Miyazawa T, et al. Worse survival after curative resection in patients with pathological stage I non-small cell lung cancer adjoining pulmonary cavity formation. J Thorac Dis 2017;9:3038-44. [Crossref] [PubMed]
- Watanabe Y, Kusumoto M, Yoshida A, et al. Surgically resected solitary cavitary lung adenocarcinoma: association between clinical, pathologic, and radiologic findings and prognosis. Ann Thorac Surg 2015;99:968-74. [Crossref] [PubMed]
- Kunihiro Y, Kobayashi T, Tanaka N, et al. High-resolution CT findings of primary lung cancer with cavitation: a comparison between adenocarcinoma and squamous cell carcinoma. Clin Radiol 2016;71:1126-31. [Crossref] [PubMed]
- Shigefuku S, Kudo Y, Yunaiyama D, et al. Prognostic factors for surgically resected non-small cell lung cancer with cavity formation. J Thorac Dis 2018;10:973-83. [Crossref] [PubMed]
- Chen C, Fu S, Ni Q, et al. Cavity Formation is a Prognostic Indicator for Pathologic Stage I Invasive Lung Adenocarcinoma of ≥3 cm in Size. Med Sci Monit 2019;25:9003-11. [Crossref] [PubMed]
- Byrne D, English JC, Atkar-Khattra S, et al. Cystic Primary Lung Cancer: Evolution of Computed Tomography Imaging Morphology Over Time. J Thorac Imaging 2021;36:373-81. [Crossref] [PubMed]
- Kojima Y, Saito H, Sakuma Y, et al. Correlations of thin-section computed tomographic, histopathological, and clinical findings of adenocarcinoma with a bubblelike appearance. J Comput Assist Tomogr 2010;34:413-7. [Crossref] [PubMed]
- Utrera Pérez E, Trinidad López C, González Carril F, et al. Can pseudocavitation in lung tumors predict the diagnosis of adenocarcinoma with lepidic growth? Radiologia 2019;61:396-404. (Engl Ed). [Crossref] [PubMed]
- Shinohara S, Sugaya M, Onitsuka T, et al. Impact of the favorable prognosis of patients with lung cancer adjoining bullae. J Thorac Dis 2018;10:3289-97. [Crossref] [PubMed]
- Hanaoka N, Tanaka F, Otake Y, et al. Primary lung carcinoma arising from emphysematous bullae. Lung Cancer 2002;38:185-91. [Crossref] [PubMed]
- Kaneda M, Tarukawa T, Watanabe F, et al. Clinical features of primary lung cancer adjoining pulmonary bulla. Interact Cardiovasc Thorac Surg 2010;10:940-4. [Crossref] [PubMed]
- Tan Y, Gao J, Wu C, et al. CT Characteristics and Pathologic Basis of Solitary Cystic Lung Cancer. Radiology 2019;291:495-501. [Crossref] [PubMed]
- Ma Z, Wang S, Zhu H, et al. Comprehensive investigation of lung cancer associated with cystic airspaces: predictive value of morphology. Eur J Cardiothorac Surg 2022;62:ezac297. [Crossref] [PubMed]
- Xue X, Wang P, Xue Q, et al. Comparative study of solitary thin-walled cavity lung cancer with computed tomography and pathological findings. Lung Cancer 2012;78:45-50. [Crossref] [PubMed]
- Qi Y, Zhang Q, Huang Y, et al. Manifestations and pathological features of solitary thin-walled cavity lung cancer observed by CT and PET/CT imaging. Oncol Lett 2014;8:285-90. [Crossref] [PubMed]
- Deng H, Zhang J, Zhao S, et al. Thin-wall cystic lung cancer: A study of 45 cases. Oncol Lett 2018;16:755-60. [Crossref] [PubMed]
- Zhang J, Deng H, Wu CC, et al. The mechanism of formation of thin-walled cystic lung cancer. Medicine (Baltimore) 2019;98:e15031. [Crossref] [PubMed]
- Pan X, Wang H, Yu H, et al. Lung cancer associated with cystic airspaces: CT and pathological features. Transl Cancer Res 2020;9:3960-4. [Crossref] [PubMed]
- Haider E, Burute N, Harish S, et al. Lung cancer associated with cystic airspaces: Characteristic morphological features on CT in a series of 11 cases. Clin Imaging 2019;56:102-7. [Crossref] [PubMed]
- Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr 2015;39:102-8. [Crossref] [PubMed]
- Yu J, Wang L, Wu J, et al. CT features in peripheral lung cancer with thin-walled cavity. Chinese Journal of Radiology 2015;49:99-102.
- Kourouni I, Abramovich CM, Tamarkin SW, et al. A perplexing airspace: peace of mind now or later. Breathe (Sheff) 2021;17:210017. [Crossref] [PubMed]
- Xue XY, Liu YX, Wang KF, et al. Computed tomography for the diagnosis of solitary thin-walled cavity lung cancer. Clin Respir J 2015;9:392-8. [Crossref] [PubMed]
- Saito H, Yamada K, Hamanaka N, et al. Initial findings and progression of lung adenocarcinoma on serial computed tomography scans. J Comput Assist Tomogr 2009;33:42-8. [Crossref] [PubMed]
- Gafoor K, Patel S, Girvin F, et al. Cavitary Lung Diseases: A Clinical-Radiologic Algorithmic Approach. Chest 2018;153:1443-65. [Crossref] [PubMed]
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol 1980;135:1269-71. [Crossref] [PubMed]
- Nin CS, de Souza VV, Alves GR, et al. Solitary lung cavities: CT findings in malignant and non-malignant disease. Clin Radiol 2016;71:1132-6. [Crossref] [PubMed]
- Giacomelli IL, Barros M, Pacini GS, et al. Multiple cavitary lung lesions on CT: imaging findings to differentiate between malignant and benign etiologies. J Bras Pneumol 2020;46:e20190024. [Crossref] [PubMed]
- Honda O, Tsubamoto M, Inoue A, et al. Pulmonary cavitary nodules on computed tomography: differentiation of malignancy and benignancy. J Comput Assist Tomogr 2007;31:943-9. [Crossref] [PubMed]
- Coffey JP, Hill JC. 18F-fluoro-2-deoxy-D-glucose standardized uptake value in cavitating non-small-cell lung carcinoma. Nucl Med Commun 2008;29:1040-5. [Crossref] [PubMed]
- Shin KE, Park JS, Lee JW. Diagnostic Accuracy of CT-Guided Core Needle Biopsy for Thin-Walled Cavitary Pulmonary Lesions. AJR Am J Roentgenol 2021;216:369-75. [Crossref] [PubMed]
- Nakahara Y, Mochiduki Y, Miyamoto Y. Percutaneous needle washing for the diagnosis of pulmonary thin-walled cavitary lesions filled with air. Intern Med 2007;46:1089-94. [Crossref] [PubMed]
- Belet U, Findik S, Ozmen Z, et al. Percutaneous cavitary lavage in the diagnosis of pulmonary cavities. J Thorac Dis 2013;5:440-5. [Crossref] [PubMed]
- Kiranantawat N, Petranović M, McDermott S, et al. Feasibility and accuracy of CT-guided percutaneous needle biopsy of cavitary pulmonary lesions. Diagn Interv Radiol 2019;25:435-41. [Crossref] [PubMed]
- Zhuang YP, Wang HY, Zhang J, et al. Diagnostic accuracy and safety of CT-guided fine needle aspiration biopsy in cavitary pulmonary lesions. Eur J Radiol 2013;82:182-6. [Crossref] [PubMed]
- Black JA, Blake MP, Cameron DC. A contrast technique to improve sampling in cavitating lung lesions. Australas Radiol 1996;40:6-9. [Crossref] [PubMed]
- Araki T, Nishino M, Gao W, et al. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax 2015;70:1156-62. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Watanabe Y, Kusumoto M, Yoshida A, et al. Cavity Wall Thickness in Solitary Cavitary Lung Adenocarcinomas Is a Prognostic Indicator. Ann Thorac Surg 2016;102:1863-71. [Crossref] [PubMed]
- Mase VJ Jr, Detterbeck FC. Approach to the Subsolid Nodule. Clin Chest Med 2020;41:99-113. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Detterbeck FC, Mase VJ Jr, Li AX, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation-part 2: systematic review of evidence regarding resection extent in generally healthy patients. J Thorac Dis 2022;14:2357-86. [Crossref] [PubMed]
- Park HS, Detterbeck FC, Madoff DC, et al. A guide for managing patients with stage I NSCLC: deciding between lobectomy, segmentectomy, wedge, SBRT and ablation-part 4: systematic review of evidence involving SBRT and ablation. J Thorac Dis 2022;14:2412-36. [Crossref] [PubMed]
- Dou P, Meng Y, Zhao H, et al. Serial CT changes in different components of lung cancer associated with cystic airspace in patients treated with neoadjuvant chemotherapy. Sci Rep 2021;11:23544. [Crossref] [PubMed]
- Parisi C, Lamberti G, Zompatori M, et al. Evolution of cystic airspaces lung lesions on immune checkpoint inhibition in non-small cell lung cancer. J Immunother Cancer 2020;8:e000502. [Crossref] [PubMed]
- Shen Y, Zhang Y, Guo Y, et al. Prognosis of lung cancer associated with cystic airspaces: A propensity score matching analysis. Lung Cancer 2021;159:111-6. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Rodríguez Alvarado I, Goicoechea Irigaray M, Gómez Hernández MT. Cystic Adenocarcinoma of the Lung. Arch Bronconeumol 2019;55:157. (Engl Ed). [Crossref] [PubMed]
- Sasaki T, Kinoshita Y, Fujita M, et al. Aggressive Cystic and Cavitary Appearances in Lung Adenocarcinoma. Intern Med 2017;56:119-20. [Crossref] [PubMed]
- Wu T, Ding Q, Yu Y, et al. Aggressive Multiple Cystic Changes in Lung Adenocarcinoma: An Unusual Presentation. Am J Med Sci 2017;354:e5. [Crossref] [PubMed]
- Wang X, Tao YX, Zhang M, et al. Solitary thin-walled cystic lung cancer with extensive extrapulmonary metastasis: A case report and review of the literature. Medicine (Baltimore) 2018;97:e12950. [Crossref] [PubMed]
- Detterbeck FC, Marom EM, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11:666-80.
- Kazerooni E, Aberle DR, Black WC, et al. Lung-RADS® 2022 American College of Radiology 2022 [November 2022]. Available online: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/Lung-RADS-2022.pdf


