Intraoperative prevention and conservative management of postoperative prolonged air leak after lung resection: a systematic review
Introduction
Prolonged or persistent air leak (PAL) after lung resection is defined as air loss from the residual lung parenchyma persisting beyond the fifth postoperative day and, according to literature, is the most common post-operative complication after pulmonary resection with an incidence ranging from 5% to 25% (1). In most cases, PAL is due to an alveolar-pleural fistula (APF), defined as communication between the parenchymal alveoli distal to a terminal bronchus with the pleural space, whereas the second main cause might be ascribed to the broncho-pleural fistula (BPF), consisting in a communication between the bronchus and pleura (2).
Risk factors are related both to the surgical procedure and to the patients’ features: the most at-risk patients are usually those who underwent major lung resection, who present incomplete interlobar fissures or pleural adhesion, and were affected by chronic obstructive pulmonary disease (COPD), emphysema, diabetes or who are under steroid therapy (3).
PAL is usually associated with an increased risk of morbidity and mortality (especially when it occurs after pneumonectomy), a longer chest tube duration, hence a prolonged hospitalization with all that it entails (4).
As reported by Cerfolio et al., severity of post-operative air leak could be classified according to the stage of the respiratory cycle in which the air leak is appreciated trough the drainage system. In this classification, air leakage severity increases over the following interval: Grade 1, air leak is appreciated only during a forced exhalation (coughing); Grade 2, expiratory phase (in spontaneous breathing), Grade 3, during the inspiration (positive pressure ventilation or in large BPF); Grade 4, both during inhalation and exhalation (5).
Most of literature, however, is focused on the potential prognostic factors leading to PAL, such as age, COPD, smoking history, pleural adhesions and upper lobe resections (6-8) rather than on its management which, in fact, has never been systematically analyzed. The main reason is the high number of measures described to prevent or solve the intra- or post-operative PAL, that each Center would rather adopt according to its availability and surgeon’s expertise and confidence (9); therefore, despite the high incidence of post-operative PAL, specific guidelines are lacking.
PAL can be prevented by adopting some intraoperative measures or treated both intraoperatively and postoperatively in many ways.
The most common intraoperative measures described in literature are the pleural tent (especially after an upper lobectomy), reinforcement or buttressing of the staple line, the fissure-less technique, and the use of biological glues or sclerosant agents. On the other hand, different authors proposed several techniques to better manage the PAL as the routinely application of suction to the chest drains, the use of digital drainage system, intrapleural blood patch or sclerosant injection (3,4). We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-736/rc).
Methods
The aim of this systematic review was to compare the efficacy of the most common adopted measures intra or post-operatively to prevent and manage the PAL caused by APF after lung resection.
Management of BPF, that represent a severe and life-threating complication, is beyond the scope of this review.
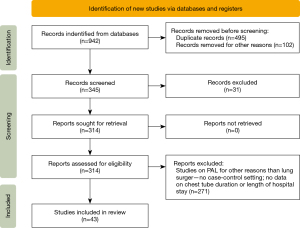
Last literature search was carried out on the 31st of March 2022. Data collection and analysis was independently conducted by two reviewers according to the PRISMA statement (http://www.prisma-statement.org/). We searched the available literature in PubMed, Cochrane Library, EMBASE, Ovid and Google Scholar, between 2000 to 2021 using “prolonged air leak” and “persistent air leak” as search terms. The reviewers assessed all articles obtained to determine whether they met our inclusion criteria. To be included, a study must have been an original article comparing the tested measure versus the standard of care. All reports on PAL due to BPF were excluded. Outcomes of interest were: reduced PAL duration, decreased APF incidence, shortened chest drain duration and postoperative length of stay. Publications were included if the following criteria were met (Figure 1):
- Patients underwent lung resection, excluded pneumonectomy.
- Original case-control studies, where the tested measure to prevent PAL was compared to the standard of care.
- At least one chest drain applied after surgery.
- Studies including ≥10 patients per group.
- Articles in English language.
Exclusion criteria were:
- Reviews/meta-analyses, case reports and book chapters.
- Reports based on national tumor registries/outcome reports (i.e., SEER, NCDB) to avoid multiple inclusion of patients.
- Patients exclusively affected or treated for pneumothorax.
- Patients undergone exclusively lung decortication or lung volume reduction surgery.
- Studies with less than 10 patients for each group.
- Reports published before the year 2000.
- Articles whose full text was not available.
- PAL due to broncho-pleural fistula.
To achieve maximum sensitivity, all search terms were combined with Boolean operators and searched as both key words and MeSH terms. Following exclusion of articles based on title or abstract, full-text articles selected had reference lists searched for any potential further articles to be included in this review.
The search Query of this study was (“prolong”[All Fields] OR “prolongation”[All Fields] OR “prolongations”[All Fields] OR “prolonged”[All Fields] OR “prolonging”[All Fields] OR “prolongs”[All Fields]) AND (“air”[MeSH Terms] OR “air”[All Fields]) AND “leak”[All Fields]. Translations: prolonged: “prolong”[All Fields] OR “prolongation”[All Fields] OR “prolongations”[All Fields] OR “prolonged”[All Fields] OR “prolonging”[All Fields] OR “prolongs”[All Fields]; air: “air”[MeSH Terms] OR “air”[All Fields].
The PICOs strategy was the following:
- Population: human adult (>18 years) patients underwent lung resection for both benign and malignant disease. During the operation and postoperatively, air leak due to APF was evaluated and managed.
- Intervention: various measures adopted to prevent PAL during surgery or to manage the air leak both intra and post-operatively.
- Comparison: experimental treatment versus standard care.
- Outcomes: reduced PAL duration or decreased APF incidence, shortened chest drain duration and postoperative hospital stay.
- Study design: only original articles, both retrospective case-control studies and prospective randomized clinical trials, were selected.
Risk of bias was assessed using the Cochrane tool (Table S1).
Results
The initial search yielded 942 studies of which 899 were excluded based on non-matching abstracts and titles. Subsequently, we identified matching references in the remaining 43 studies and repeated this process iteratively until no new, matching studies could be identified.
Studies selected were divided in blocks according to the timing (intra- and post-operative) and the kind of the measure adopted: reinforced staplers, sealants, fat pads apposition, fissure-less technique for lobectomy, pleural tenting and others intra-operative invasive techniques; autologous blood patch or chemical pleurodesis, and chest (digital) drain management for what concerns post-operative methods.
For each study, we reported in Table 1 and Table 2 the following features: name of the first author and the publication year, study design, population of the experimental and the control groups, outcomes on PAL, chest drain duration and hospitalization, types of lung resection performed, and other relevant data if available.
Table 1
| Author, year | Used method | Study design | Groups | Number of patients | Mean AL/chest drain/LOS duration | Morbidity/mortality | Type of lung resection | Conclusions/notes |
|---|---|---|---|---|---|---|---|---|
| Reinforced staplers | ||||||||
| Miller, 2001 (10) | Buttressed stapler (bovine pericardium) | Multicentric RCT | Buttressed stapler vs. ST | 40 vs. 40 | POAL: 2 vs. 3 days (P=0.27); Chest tube: 5.9 vs. 6.3 days (P=0.6); LOS: 8 vs. 9 days (P=0.24) | Morbidity 17.5% vs. 12.5% (not significant); mortality: 5% vs. 0 (P=0.15) | Lobectomy: 65; segmentectomy: 15 | Mean FEV1 =1.88 vs. 1.93 L; no differences between the two groups, even if the buttressed staplers’ group shows a trend toward shortened air leak time and earlier tube removal |
| Deguchi, 2020 (11) | Polyglycolic acid (PGA) reinforced stapler | Retrospective | Stapler vs. PGA-stapler | 125 vs. 125 | Chest tube: 5.2 vs. 4.9 days (P=0.201); LOS: 10.6 vs. 11.5 days (P=0.386) | No differences between the two groups | Lobectomy: 125 vs.125 | PGA-staplers group’s patients are significantly more affected by respiratory diseases (COPD, interstitial pneumonia), but the Authors level out the difference by analyzing propensity-score matched groups; POAL incidence 12.5% vs. 25.2% (P<0.001); use of PGA-staplers is identified as an independent factor for preventing POAL (OR =0.38, P=0.015) |
| Sealants | ||||||||
| Allen, 2004 (12) | Biodegradable polymeric sealant | Multicentric RCT | Sealant group vs. control group | 103 vs. 58 | POAL: 2 days in both groups (P=0.410); chest drain: 6.8 vs. 6.2 days (P=0.679); LOS: 6 vs. 7 days (P=0.028) | No differences between the two groups; no sealant-related complications | Bilobectomy: 4 vs. 1; lobectomy: 70 vs. 41; segmentectomy: 5 vs. 4; wedge resection: 20 vs. 9 | COPD incidence is comparable between the two groups (34% vs. 27.6%); 35% of patients in the sealant group are POAL-free vs. 14% of the control group (P=0.005); number and sources of IOAL affects outcomes |
| D'Andrilli, 2009 (13) | CoSeal® | RCT | CoSeal®vs. ST | 102 vs. 101 | POAL: 3.5 vs. 4.2 days (P=0.01); LOS: 5.7 vs. 6.2 days (P=0.18) | No sealant-related adverse events; morbidity rate: 2% vs. 3% (P=0.64) | Bilobectomy: 3 vs. 3; lobectomy: 60 vs. 44; segmentectomy/wedge: 38 vs. 53; sleeve lobectomy: 1 vs. 1 | COPD: 13.7% vs. 17.8%; smoking history: 59.8% vs. 62.4%; AL POD1: 19.6 vs. 4.6% (P=0.001); POD2: 23.5 vs. 41.6% (P=0.006). CoSeal® is safe, effective and superior to ST |
| De Leyn, 2010 (14) | PleuraSeal® | Multicentric RCT | PleuraSeal®group vs. ST | 62 vs. 59 | Chest drain: 93.7 vs. 94.1 hours (P=0.559); LOS: 312 vs. 288 hours (P=0.292) | No differences between the two groups; no sealant-related complications | Lobectomy: 61 vs. 58; segmentectomy: 1 vs. 1 | COPD: 46.8% vs. 33.9% (P=0.195); emphysema: 25.8% vs. 15.3% (P=0.181); smoking history: 75.5% vs. 86.4% (P=0.719); IOAL successful sealing: 71% vs. 23.7% (P<0.001); POAL: 4.8% vs. 11.9% (P=0.303); in patients with clinically significant air leaks (grade 2 and 3), significantly fewer patients have postoperative air leaks when treated with the pleural sealant |
| Ueda, 2010 (15) | PGA and fibrin glue | Retrospective | Mesh-and-glue (MG) vs. glue alone (G) | 61 vs. 61 | Chest drain: 1.1 vs. 3 days (P<0.001)LOS: 8.7 vs. 11 days (P=0.007) | No differences between the two groups; no sealant-related complications | Not reported | Intraoperative air leak: 62% of both groups. Emphysema index >10%: 25% of G group; 20% of MG group; authors recommend the use of the combination PGA + fibrin glue especially in patients undergoing upper lobectomies and with severe emphysema |
| Tan, 2011 (16) | CoSeal® | RCT | CoSeal®vs. ST | 60 vs. 59 | POAL: P=0.09;Chest drain: 4 vs. 3 daysLOS: 7 vs. 6 days | AL POD1: 61 vs. 63%; POD2: 49 vs. 39% | Lobectomy: 44 vs. 50; bilobectomy: 7 vs. 5; wedge resection: 4 vs. 5; lobectomy + wedge: 5 vs. 1 | Median FEV1: 2.0 vs. 1.96 L/min; authors don’t recommend CoSeal® routinary use |
| Yano, 2012 (17) | PGA and fibrin glue | Retrospective | Mesh-and-glue (MG) vs. glue alone (G) | 173 vs. 204 | Chest drain: 2.7 vs. 4.2 days (P<0.01) | Not reported | Lobectomy:120 vs. 158; segmentectomy: 10 vs. 9; wedge resection: 43 vs. 37 | IOAL rate: not reported; POAL incidence 0 vs. 6.6% (P<0.01); COPD rates: 25.8% vs. 23.7% (P=0.67) |
| Lequaglie, 2012 (18) | CoSeal® | RCT | CoSeal®vs. ST | 111 vs. 105 | LOS: 4.3 vs. 8.4 days (P=0.0001) | Not reported | Lobectomy: 74 vs. 76; wedge resection: 34 vs. 25 | FEV1 values are comparable between the two groups; PAL incidence:2.7% vs. 11.4% (P=0.0013); study also included decortications; CoSeal® is an effective method of reducing postoperative alveolar air leaks |
| Klijian, 2012 (19) | Progel® (polymeric biodegradable hydrogel sealant) | Retrospective | PSG: pleural-sealant group; CG: control group | 36 vs. 34 | Chest tube: 1 vs. 2.5 days (P<0.0001) LOS: 1.5 vs. 3 days (P=0.047) |
No differences between the 2 groups | Lobectomy: 8 vs. 11; wedge resection: 24 vs. 30; segmentectomy/bisegmentectomy: 9 vs. 2 | COPD and emphysema prevalence are similar in both groups; study also included decortications and bullectomies; in the sealant’s group there are 5 patients affected by PNX (0 in the control group); POAL incidence: 11% vs. 58.8% (P<0.0001) |
| Petrella, 2016 (20) | Enable-Innoseal TP4® (Cyanoacrilate + vitamin E) for air leak grade 1/2 | RCT | Innoseal®vs. standard aerostasis | 30 vs. 30 | Chest drain: 3.5 vs. 5 days (P=0.005) | 10% (6.6% minor, 3.3% major: BPF) | Anatomic resection (lobectomy, bilobectomy, or typical segmentectomy): 12 vs. 12; non anatomic resection (wedge resection, tumorectomy): 18 vs. 18 | The case-control population is matched 1:1 according to surgical procedure and the male/female ratio, median age, and median preoperative FEV1; Innoseal® group: earlier drain removal; biases: good pulmonary function and grade 1 air leak in the majority of patients, non-randomized, inclusion of both anatomical and wedge lung resections |
| Gologorsky, 2019 (21) | Progel® on staple lines | Retrospective | Progel® group vs. control group | 84 vs. 92 | Chest drain: 23.5 vs. 23 hours (P=0.721) | 1% in both groups | Wedge resection: 84 vs. 92 | COPD rates: 14.29% vs. 13.33% (P=0.856); no differences between the two groups in terms of POAL rate (23.81% vs. 17.39%, P=0.33); progel does not improve POAL and LOS |
| Porrello, 2019 (22) | Fibrin glue | RCT | Glue arm vs. Control group | 90 vs. 99 | Chest drain: 4.15 vs. 4.45 days (not significant); LOS: 7.4 vs. 9.1 (P=0.010) | Not reported | Lobectomy/bilobectomy: 90 vs. 90 | Mean FEV1: 85% vs. 88%; PAL incidence: 1.1% vs. 8.1% (P=0.025) |
| Kawashima, 2020 (23) | Autologous fibrin glue (AFG) | Retrospective | Partially AFG (PAFG); completely AFG (CAFG); non-AFG | 83 vs. 94 vs. 30 | Chest tube: 3.23 vs. 3.16 vs. 3.17 days (P=0.405)LOS: 6.45 vs. 7.97 vs. 7.40 days (P=0.604) | No AFG or non-AFG-related complications | Lobectomy:48 vs. 54 vs. 16 Segmentectomy: 35 vs. 40 vs. 14 |
Method: 1–2 mL of AFG + PGA sheet applied to the exposed parenchyma; there are no statistically significant differences among the three groups regarding smoking habit, COPD or interstitial pneumonia; however, patients in the non-AFG group tend to be older, and have higher rates of COPD and interstitial pneumonia compared with the PAFG and CAFG groups; incidence of PAL: 13.3% vs. 12.8% vs. 16.7% (P=0.821); no differences in terms of costs; AFGs could be a viable alternative to conventional allogenic fibrin glues |
| Anegg, 2006 (24) | TachoSil® | RCT | TachoSil®vs. ST for air leak grade 1/2 | 75 vs. 77 | Chest drain: 5.1 vs. 6.3 days (P=0.022); LOS: 6.2 vs. 7.7 days (P=0.01) | No TachoSil®-related complications; similar morbidity rate in both groups | Lobectomy: 68 vs. 68; segmentectomy: 10 vs. 9 | AL POD1: 43.6 vs. 86.1% (P=0.004); POD2: 20.13 vs. 42.46% (P=0.023); AL >48 h: no significant differences between the two groups. TachoSil® is safe, effective and superior to ST |
| Rena, 2009 (25) | TachoSil® | RCT | Stapler group vs. electrocautery dissection + TachoSil® group | 30 vs. 30 | POAL: 4.3 vs. 1.6 days (P=0.0018)Chest drain: 5.9 vs. 3.5 days (P=0.0021)LOS: 7.5 vs. 5.87 days (P=0.01) | No differences between the two groups. No stapler/TachoSil® related complications | Upper lobectomy: 30 vs. 30 | Pilot trial on COPD patients scheduled for upper lobectomy. The two groups are comparable in terms of respiratory function test parameters. Incidence of POAL: 96% vs. 55% (P=0.03) |
| Marta, 2010 (26) | TachoSil® | Multicentric RCT | TachoSil®vs. ST for mild air leak | 148 vs. 151 | AL: better in the TachoSil group (P=0.030); chest drain: 4 vs. 5 days (P=0.054); LOS: 8 vs. 9 days (P=0.35) | Similar in both groups with exception for atrial fibrillation (11 vs. 5 patients) | Lobectomy: 148 vs. 151 | The two groups are comparable in terms of respiratory function test parameters or smoking habit. TachoSil is safe, effective and superior to ST. High between-centers variability |
| Fat pads apposition | ||||||||
| Yoshimura, 2002 (27) | Fibrin glue + pedicled pericardial fat pad | RCT | A: fibrin glue; B: fibrin glue+ pericardial fat pad | 15 vs. 15 | POAL: 4.8 vs. 3.6 days (P=0.4233); chest drain: 8.6 vs. 8.4 days (P=0.9021) | No procedure-related adverse events | Lobectomy: 9 vs. 12; segmentectomy: 6 vs. 3 | The two groups are comparable in terms of respiratory function test parameters; POAL cessation within 24 h: 6.6% vs. 40% (P=0.0309); the pedicled pericardial fat pad is safe and effective as an additional sealant to stop IOAL |
| Shintani, 2015 (28) | Subcutaneous fat pad | Retrospective | S: fibrin glue + absorbable PGA mesh sheet for superficial air leak; F: subcutaneous fat pad + fibrin glue + sutures for deep air leak | 100 vs. 66 | POAL: 2.7 vs. 1.4 days (P=0.015); chest drain: 5.8 vs. 4 days (P=0.002) | Not reported | Lobectomy: 68 vs. 49; segmentectomy: 32 vs. 17 | Mean Tiffeneau index: 74% vs. 75% (P=0.64); PAL incidence: 15% vs. 7% (P=0.15); re-operation for PAL: 2% vs. 0; the proposed method is useful for IOAL repair |
| Fissureless Technique | ||||||||
| Stamenovic, 2015 (29) | Fissureless technique | Retrospective | 1: conventional VATS lobectomy; 2: fissureless | 24 vs. 30 | Chest drain: 7.1 vs. 4.2 days (P=0.028); LOS: 12.7 vs. 8.9 days (P=0.020) | No differences in postoperative complications between the two groups (P=0.15). Postoperative complications are more present in patients with PAL (P=0.013) | Lobectomy: 24 vs. 30 | Mean FEV1: 80% vs. 76% (P=0.57); incidence of POAL and PAL > in Group 1 (P=0.004 and P=0.003); Predictors of PAL: age, ASA score, surgical technique, operation time, preoperative chemotherapy. FEV1 values did not impact on IOAL or PAL; fissureless technique is superior to conventional VATS lobectomy in preventing PAL and reducing LOS |
| Murakami, 2021 (30) | Fissureless technique for VATS right upper lobectomy | Retrospective | A: fissureless; B: traditional technique | 54 vs. 159 | POAL: 1 vs. 1.7 days (P=0.047); chest drain: 3.9 vs. 4.8 days (P=0.017) | A =11%; B =24%. No mortality. No need for reoperation. No PNX after chest drain removal | All patients underwent right upper lobectomy | The two groups are comparable in terms of FEV1 or emphysema prevalence; POAL incidence: 5.6% vs. 17% (P=0.037); greater fissural grade is associated with prolonged air leak |
| Pleural tenting | ||||||||
| Brunelli, 2002 (31) | Pleural tenting for upper lobectomies | RCT | Pleural tenting vs. no pleural tenting | 100 vs. 100 | POAL: 2.5 vs. 7.2 days (P<0.0001); LOS: 8.2 vs. 11.6 days (P<0.0001) | No differences between the two groups | Upper lobectomy: 100 vs. 100 | The two groups are comparable in terms of respiratory function test parameters and COPD prevalence; PAL incidence: 14% vs. 32% (P=0.003); pleural tenting is a safe procedure that reduces AL duration |
| Allama, 2010 (32) | Pleural tenting after upper lobectomy/bilobectomy | RCT | Pleural tenting vs. no pleural tenting | 23 vs. 25 (excluded from analysis: 3 vs. 1) | POAL: 2.96 vs. 4.68 days (P=0.015); chest tube: 4.6 vs. 5.6 days (P=0.11); LOS: 4.96 vs. 5.7 days (P=0.05) | No differences between the two groups | Upper lobectomy: 23 vs. 25 (excluded from analysis: 3 vs. 1) | Mean FEV1 80.3 l vs. 77.4 l (P=0.14); COPD prevalence: 30% vs. 40% (P=0.49); PAL incidence: 9% vs. 40% (P=0.02); factors affecting PAL incidence: COPD, adhesions, intraoperative air leak from lower lobes, pleural tent; pleural tenting is safe and useful |
| Okur, 2015 (33) | Pleural tenting after upper lobectomies/bilobectomies | RCT | Pleural tenting vs. no pleural tenting | 20 vs. 20 | Chest drain: 4.3 vs. 7.4 days (P<0.0001); LOS: 7.6 vs. 9.35 days (P=0.024) | 15% of the non-tented group needed apical drainage insertion for residual space. No tenting-related complications | Lobectomy: 16 vs. 19; bilobectomy: 4 vs. 1 | The two groups are comparable in terms of respiratory function test parameters; operative time for pleural tenting: 5–10 min |
| Miscellaneous | ||||||||
| Marulli, 2013 (34) | Laser system to complete fissure | RCT | S (staplers) vs. L (laser) | 22 vs. 22 | POAL: 3.6 vs. 2.1 days (P=0.98); chest drain: 6.3 vs. 6.4 days (P=0.44) | 77.3% vs. 36.4% (P=0.006); no laser-related adverse effects | Lobectomy: 22 vs. 22 | A Thulium laser 2,010 nm (Cyber TM, Quanta System, Italy) is used at power of 40 W; the two groups are comparable in terms of respiratory function test parameters or smoking habit; operative time 158 vs. 197 min (P=0.004); significantly lower costs for L group; aero-haemostatic laser properties allow a safe application during pulmonary lobectomy |
| Pan, 2017 (35) | Cryoneuroablation of phrenic nerve | RCT | Cryoneuroablation of PN vs. standard treatment | 104 vs. 103 | Chest drain: 3.2 vs. 4.3 (P<0.01); LOS: 7.8 vs. 8.2 (P=0.486) | No differences between the two groups | Lobectomy: 95 vs. 91; bilobectomy: 9 vs. 12 | The two groups are comparable in terms of COPD prevalence (P=0.597), smoking habit (P=590) and FEV1 (P=0.435); PAL 1.9% vs. 8.7 % (P=0.023); operative time for PN cryoablation: 5 min; reversible diaphragmatic paralysis after 1–2 months |
| Decaluwe, 2015 (36) | Tunnel technique for fissure-first lobectomy in uncomplete fissure lung | Retrospective | Fissure-first (FF) vs. hilum-first (HF) | 198 vs. 45 | Chest drain: 5 vs. 6.9 days (P=0.010); LOS: 8.3 vs. 9.7 days (P=0.165) | Major intraoperative complications: 6.7% vs. 1%; conversion rates: 4% vs. 8% | Segmentectomy: 7.6% vs. 2.2%; lobectomy: 86.4% vs. 97.8%; bilobectomy: 5.6% vs. 0% | All procedures are carried out by triportal VATS. No data on fissure completeness; mean FEV1: 89% vs. 90%; frequency of PAL: 13.2% vs. 21.4% (P=0.172); FF lobectomies are feasible and the technique is non-inferior compared to the HF technique |
ABP, autologous blood patch; AL, air leak; BPF, broncho-pleural fistula; COPD, chronic obstructive pulmonary disease; DD, digital drainage; FEV1, forced expiratory volume in 1 second; IOAL, intra-operative air leak; LOS, length of stay; PAL, prolonged air leak (>5 days); PN, phrenic nerve; PNX, pneumothorax; POAL, post-operative air leak; POD, post-operative day; RCT, randomized controlled trial; ST, standard treatment.
Table 2
| Author, year | Used method | Study design | Groups | Number of patients | Mean AL/chest drain/LOS duration | Morbidity/mortality | Type of lung resection | Conclusions/notes |
|---|---|---|---|---|---|---|---|---|
| Autologous blood patch | ||||||||
| Shackclot, 2006 (37) | Autologous blood patch | RCT | ABP vs. no ABP | 10 vs. 10 | POAL: 5 vs. 11 days (P<0.001); Chest drain: 6.5 vs. 12 days (P<0.001); LOS: 8 vs. 13.5 days (P<0.001) | 1 patient (10%) in the study group developed empyema on 7th POD, successfully treated by drainage and antibiotics | Lobectomy: 10 vs. 10 | The two groups are comparable for smoking habit (P=1.0) and presence of underlying lung disease (P=1.0); ABP can be repeated every 48 h until the AL stops; after ABP, the AL was sealed by the next day in 58.6% of treatments; ABP is effective in sealing AL after lobectomy |
| Andreetti, 2007 (38) | Autologous blood patch | RCT | A: 50 mL, B: 100 mL, C: no ABP | A: 12, B: 13, C: 15 | POAL: 2.3 vs. 1.5 vs. 6.3 days (p<0.05) | None | Lobectomy: 12 vs. 13 | Mean FEV1: 2.5 vs. 2.4 vs. 2.3 L; 100% success rate. No costs |
| Campisi, 2021 (39) | Autologous blood patch | Retrospective multicentric | A= ABP for POAL >5 days; B= observation | 109 vs. 109 | Chest drain: 8.12 vs. 9.30 days (P=0.004) LOS: 10 vs. 11 (P=0.045) | No ABP-related adverse events | Lobectomy:109 vs. 109 | The two groups are comparable for smoking habit (P=0.491) and COPD prevalence (P=0.278). Those variables are not associated to chest removal timing at univariate analysis; ABP is associated with fewer postoperative complications (6 vs. 17, P=0.015) and need for reoperation (0 vs. 4, P=0.044); 120 mL of blood are better than 60 mL |
| Hasan, 2021 (40) | Autologous blood patch | Retrospective | ABP (90 mL) vs. no ABP for POAL >5 days | 34 vs. 76 | Chest drain: 11 vs. 16 days (P=0.14); LOS (P=0.13) | No differences. Empyema < in ABP group | Wedge resection: 9 vs. 18; segmentectomy: 1 vs. 7; lobectomy: 18 vs. 44; combined: 6 vs. 7 | No significant differences between the two groups in terms of FEV1 (P=0.17) and smoking history (P=0.88); ABP is associated with a lower readmission rate (P=0.02) and reoperation (P=0.05); ABP patients are less likely to be discharged with a chest tube |
| Chemical pleurodesis | ||||||||
| Liberman, 2010 (4) | Chemical pleurodesis by talc, bleomycin, doxycycline, minocycline | Retrospective | Observation vs. pleurodesis | 33 vs. 41 | POAL: 10.7 days (all cohort); in patients underwent pleurodesis, AL ceased after a mean of 2.8 days after the procedure | No adverse event related to pleurodesis. One patient in the pleurodesis cohort developed empyema | Lobectomy: 69; bilobectomy: 5 | Differences between respiratory function tests parameters and respiratory comorbidities are not reported; pleurodesis successful in 40 of 41 patients (97.6%), 5 patients required repeated sclerosis; chemical pleurodesis is a simple, effective, and a rapid method of treating prolonged air leak after pulmonary resection |
| Jabłoński, 2018 (41) | Chemical pleurodesis by Iodine or Doxycycline | RCT | Iodine group; doxycycline group; drainage alone group | 30 vs. 34 vs. 35 | Chest drain: 9.23 vs. 11.5 vs. 13.09 days (P<0.0001); LOS: 12.67 vs. 16.5 vs. 15.89 days (significantly better in Iodine group) | No differences between the three groups. Few allergic reactions to doxycycline (1 patient) and Iodine (2 patients) | Wedge resection: 3 vs. 4 vs. 5; segmentectomy: 1 vs. 2 vs. 1; lobectomy: 24 vs. 25 vs. 26; sleeve lobectomy: 1 vs. 0 vs. 1; lower bilobectomy: 1 vs. 2 vs. 1; upper bilobectomy: 0 vs. 1 vs. 1 | Data on respiratory function tests parameters and respiratory comorbidities are not reported; pleurodesis was performed in the 6th, 7th and 8th POD in patients with PAL; PNX recurrence rate is similar between the three groups (P=0.42); iodine pleurodesis showed favorable results compared with Doxycycline pleurodesis or drainage alone |
| Chaari, 2021 (42) | Chemical pleurodesis by povidone iodine for PAL | RCT | A: povidone iodine; B: no povidone iodine | 19 vs. 21 | Chest drain: 9.21 vs. 15.62 days (P=0.001); LOS: 11.05 vs. 18.9 days (P<0.0001) | 21% (4 patients) vs. 19% (4 patients); povidone iodine-related adverse events (Group A): mild fever, chest pain, bad taste sensation; Group B: lung atelectasis, wound infection, respiratory distress, pleural empyema | Lobectomy 4 vs. 6; segmentectomy 1 vs. 0; wedge resection: 2 vs. 1 | Group A and B are similar in terms of respiratory diseases prevalence (emphysema and COPD) and smoking habit; mean number of injections per patient: 2.11; no recurrence of PNX in Group A (effectiveness: 100%), 1 in Group B (4.76%); the study includes also patients underwent bullectomy and/or pleurodesis (10 vs. 9), lung decortication (3 vs. 6) and surgery for hydatid cyst (1 vs. 3) |
| Chest drain management | ||||||||
| Brunelli, 2004 (43) | Suction | RCT | Suction −20 cmH2O vs. water seal | 73 vs. 72 | POAL: 6.1 vs. 8.0 days (P=0.9); chest drain: 10.0 vs. 12.5 days (P=0.9); LOS: 10.9 vs. 11.3 (P=0.9) | 17.8% vs. 31.9% (P=0.056). Water seal group had higher incidence of pneumonia and arrhythmia | Lobectomy: 73 vs. 72 | The two groups are comparable in terms of respiratory function tests parameters, smoking habit and pleural adhesions prevalence; incidence of PAL: 30.1% vs. 27.8% (P=0.8); no significant differences even when corrected for length of stapled parenchyma and site of resection; authors routinely perform pleural tenting in upper lobectomies/bilobectomies |
| Alphonso, 2005 (44) | Suction | RCT | Suction vs. no suction | 116 vs. 123 | AL for more than 6 days: 7.8% vs. 10.1% | Recurrent PNX: 2 cases vs. 3 cases | Wedge resection: 18 vs. 19; lobectomy: 55 vs. 56; lung biopsy: 10 vs. 11 | In both groups there were current (42 vs. 49) and ex-smokers (50 vs. 55). No data on respiratory function tests or lung diseases’ prevalence are available; no differences between the two groups in terms of persistence of AL (P=0.62); authors are in favor of no-suction policy; study comprehends also patients underwent surgical pleurodesis for pneumothorax (33 vs. 37) and lung biopsies (10 vs. 11) |
| Brunelli, 2005 (45) | Alternative suction | RCT | Water seal vs. alternate suction | 47 vs. 47 | POAL: 4.2 vs. 3.1 days (P=0.3); chest drain: 8.6 vs. 6.2 days (P=0.002); LOS 10.4 vs. 8 days (P=0.004) | No differences between the two groups | Lobectomy: 47 vs. 47 | The two groups are comparable in terms of respiratory function tests parameters, smoking habit and pleural adhesions prevalence; alternate suction patients showed a reduced incidence of POAL >4 days (P=0.04) and >7 days (P=0.02); suction applied overnight allows early mobilization of patients |
| Brunelli, 2013 (46) | Suction | RCT | Group 1: regulated individualized suction (range: −11 to −20 cmH2O, according to lobectomy type); Group 2: regulated seal (−2 cmH2O) | 50 vs. 50 | POAL: 28 vs. 22.2 h (P=0.6) in the whole cohort; between those having POAL immediately after extubation, patients of Group 2 had an air leak lasting 34.5 h less than those of Group 1 (52.9 vs. 87.4 h, P=0.07) | No differences between the two groups | Lobectomy: 50 vs. 50 | Group 1 patients have significantly higher mean Tiffeneau Index value if compared to Group 2 (0.74 vs. 0.69, P=0.006); nevertheless, other respiratory function test parameters are comparable between the two groups, as well as the prevalence of pleural adhesions; PAL incidence: 5 vs. 4 patients (P=0.7); the regulated seal mode had the same effect as the regulated suction one; patients with immediate POAL managed with regulated seal showed a trend towards a shorter duration of air leak |
| Holbek, 2019 (47) | Suction | RCT | −2 vs. −10 cmH2O | 111 vs. 111 | Chest drain: 27.4 vs. 47.5 h (P=0.047); LOS: 2 vs. 3 days (P=0.18) | No differences in the proportion or the size of the PNX or subcutaneous emphysema after drain removal, nor in postoperative morbidity | Lobectomy: 111 vs. 111 | Mean FEV1: 87.1% of expected vs. 87.4%; mean Tiffeneau Index value: 68% of expected vs. 71.3%; current or ex-smoker status: 103 vs. 97; COPD prevalence: 57 patients vs. 48; previous ipsilateral surgery: 12 patients vs. 6; incidence of PAL >5 days: 14.4% vs. 24.3% (P=0.089); a low suction level significantly shortened time to air leak cessation and total fluid production |
| Mitsui, 2021 (48) | Suction | Retrospective | A (−5 cmH2O); B (−10 cmH2O); C (−20 cmH2O) | 49 vs. 100 vs. 68 | POAL: 0.57 days A, 0.78 days B, 1.13 days C (P=0.019; P=0.010 for anatomical resections only) | Not reported | Wedge resection:20 vs. 53 vs. 34; segmentectomy: 4 vs. 17 vs. 4; lobectomy: 25 vs. 30 vs. 30 | Study included also patients who underwent surgery for PNX; patients with emphysema/interstitial pneumoniae: 8/2 vs. 9/4 vs. 7/2; IOAL: 45% A, 36% B, 29% C; POAL: 16% A, 24% B, 35% C; low-pressure suction after pulmonary resection seems to avoid or promptly improve postoperative air leaks |
| Digital drainage systems | ||||||||
| Filosso, 2015 (49) | Digital drainage | RCT | DDs vs. TDs | 40 vs. 40 | Chest tube: 3 vs. 5 days (P=0.0009); LOS: 7 vs. 8 days (P=0.0385) | Not reported. | Wedge/segmentectomy: 6 vs. 8; lobectomy 32 vs. 31; bilobectomy: 2 vs. 1 | FEV1 <80% of expected: 10 vs. 10 patients (P=1). Smokers: 24 vs. 25 (P=0.818); TDs were connected to wall suction; DDs were settled to maintain a constant negative pressure; DDs reduce the interobserver variability on AL quantification and allow early patient mobilization |
| Gilbert, 2015 (50) | Digital drainage | RCT | DDs vs. TDs | 43 vs. 42 | LOS: 6 vs. 6 days (P=0.36)Chest tube: 4.9 vs. 5.6 days (P=0.11) | Chest tube reinsertions occurred only in patients randomized to TDs | Lobectomy: 31 vs. 37 | The two groups are comparable in terms of comorbidities, lung diseases prevalence, smoking habit, pleural adhesions and intraoperative use of sealants; clamping trials: 23% vs. 50% (P=0.01): digital technology can alter chest tube management by significantly reducing clamping trials before removal of the chest tube |
| Mendogni, 2021 (51) | Digital drainage | Multicentric RCT | DDs vs. TDs | 94 vs. 115 | Chest tube: 2.4 vs. 3.8 days (P=0.203); difference between LOS and chest tube duration: 1.3 vs. 1.4 days (P=0.999) | No differences between the two groups | Lobectomy: 94 vs. 115 | Interim analysis of RCT’s preliminary data; COPD: 15 vs. 12 patients (P=0.17). Asthma: 3 vs. 3 patients (P=0.04). Respiratory function test values are comparable in the two groups; incidence of PAL: 1.1% vs. 2.6% (P=0.999); presence of AL in the 1st POD predicts the prolonged chest tube requirement |
ABP, autologous blood patch; AL, air leak; BPF, broncho-pleural fistula; COPD, chronic obstructive pulmonary disease; DD, digital drainage; FEV1, forced expiratory volume in 1 second; IOAL, intra-operative air leak; LOS, length of stay; PAL, prolonged air leak (>5 days); PN, phrenic nerve; PNX, pneumothorax; POAL, post-operative air leak; POD, post-operative day; RCT, randomized controlled trial; ST, standard treatment; TD, traditional drainage.
Intraoperative prevention of PAL and management of APF
In Table 1 we reported the main features of the selected studies that compared intraoperative measures to prevent postoperative air leak (POAL) or seal APF, dividing them according to the technique adopted.
Miller and Deguchi tested reinforced staplers with bovine pericardium and polyglycolic acid, respectively, to complete lung resection preventing potential APF. Both authors, however, did not report statistically significant benefits in terms of air leak duration or hospitalization from the use of such buttressed staplers (10,11).
Literature reports dozens and dozens of sealants to prevent PAL after lung resection (12,13,16,18-26). Most studies, however, were case series or conducted on small cohorts of patients.
By selecting the most structured studies, we noted fluctuating results with no clear and unequivocal recommendations, although Coseal® (13,16,18), Progel® (19,21) and TachoSil® (24-26) have been proven to significantly reduce post-operative PAL incidence and duration as well as the hospitalization in most studies in which they were used. Conversely, a multicentric randomized study performed by De Leyn and colleagues in 2010 on PleuraSeal®, a synthetic biodegradable hydrogel, showed to be effective in sealing intraoperative air leak sources (71% of success rate), especially in case of Grade 2 and 3 leaks, but its use was not associated to shorter chest drain maintenance or length of stay (14).
Two retrospective studies described the results obtained by combining polyglycolic acid sheets with fibrin glue and reported significant shorter chest tube and hospital stay in the group treated with mesh and glue if compared to the group treated with the glue alone. Noticeably, the groups of these studies were comparable in terms of COPD incidence and surgical procedures performed (15,17).
Anyway, none of these studies reported sealant application-related complications.
Yoshimura and Shintani reported the application of autologous fat pads on intraoperative detected APF, with satisfying results in terms of reduced air leak incidence and chest tube duration (27,28).
Stamenovic and Murakami described a different surgical technique to prevent APF and its consequence, named fissure-less technique (29,30). According to the Authors, working on the interlobar fissure at the end of the surgical operation, reduces the APF incidence without increasing postoperative morbidity and surgery time.
Brunelli, Allama and Okur, instead, reported a different and efficient technique to prevent air leak consisting in the pleural tenting after upper lobectomies, which aims to reduce the residual pleural space (31-33).
Lastly, we reported the use of less common measures described by Marulli, Pan and Decaluwe who experienced novel techniques to prevent PAL by using Thelium laser system to complete fissures, cryoneuroablation of phrenic nerve to reduce the residual space after lung resection and the tunnel technique for fissure-first lobectomy in uncomplete fissure patients, respectively (34-36).
Postoperative PAL conservative management
In Table 2, the principal postoperative air leak control methods are reported.
Autologous blood patch (ABP) is one of the most worldwide used technique to seal APF, since it has many advantages: it can be performed bedside and repeated about every 48 h, it has no risks of allergic reactions and low risk of adverse events or complications (37-39). It consists in the administration, through the chest tube, of 50–120 mL of autologous venous blood. After that, the chest tube is elevated above the level of its insertion in the thorax to avoid the early reflux of the blood, and the patient should turn on his/her sides every 15 min for about 2 h to allow the blood to distribute uniformly in the pleural space. Many Authors reports this method as safe and effective in stopping air leakage, even if some precautions must be taken: first of all, the maneuvers have to be performed without compromising sterility to avoid infections. In the reports examined, however, only Shackclot et al. in 2006 reported a case of empyema after ABP (37). In another study performed by Hasan and colleagues in 2021, conversely, empyema was less encountered in the ABP group (40). Moreover, Andreetti et al. recommended, in their randomized controlled trial published in 2007 (38), to use 100 mL of autologous blood, since patients undergone ABP with 50 mL of blood met longer mean air losses (2.3 vs. 1.5 days). Similarly, Campisi and his equip stated in 2021 (39) that 120 mL of blood is more effective than 60 mL in reducing chest drain duration, hospitalization, postoperative morbidity and need for reoperation.
Other than the autologous blood patch, also the intrapleural instillation of fresh frozen plasma has been successfully used by some Authors to treat postoperative PAL. This method is still not standardized, even if it is reported as feasible and safe (52,53). Unfortunately, no case-control studies or RCT have been performed to assess the actual effectiveness of this technique.
Pleurodesis can also be induced chemically using various agents, among which the more known are Povidone Iodine and Doxycycline. In a prospective randomized study of 2018, Jabłoński et al. compared the use of Povidone Iodine, Doxycycline (administered in 6th, 7th and 8th postoperative day) and water seal alone for patients affected by PAL (41). They reported that Iodine pleurodesis showed favorable results compared with Doxycycline pleurodesis or drainage alone, since POAL and LOS was significantly shorter in the first group, at the cost of slightly increased thoracic pain and few allergic reactions (6%). Also, Chaari and colleagues (42) evaluated the role of Povidone Iodine injection to treat PAL, finding out that after a mean number of 2.11 treatments per patient, drainage period and LOS were significantly shorter if compared to those of untreated patients. In addition, morbidity in the group of untreated patients was more severe, including infections and respiratory distress probably due to persistently incomplete lung re-expansion.
Talc pleurodesis has rarely been used for PAL. Its application is described in a 2010 retrospective study performed by Liberman et al. (4), in which the Authors evaluated the effectiveness of various agents (talc: 30 patients, bleomycin: 1 patient, doxycycline: 7 patients, minocycline: 1 patient) in a cohort of 78 patients underwent lung resections (mostly lobectomies) with postoperative PAL. They reported a pleurodesis success rate of 97.6% and concluded that sclerosis is a simple and effective treatment of PAL.
Other authors focused on chest drainage management to overcome the problem of postoperative air leak: suction application, timing and negative pressure, and the benefit from the use of digital systems are still uncodified. Brunelli and colleagues wrote three papers since 2004 on this topic (43,45,46), concluding that an alternative (applied overnight) individualized regulated (range, −11 to −20 cmH2O) suction can be applied to allow patients’ mobilization during the daytime and it seems to be associated to a shorter air leak duration if compared to water seal; however, their findings did not reach the statistical significance. Alphonso et al. in their paper written in 2005 (44) declared to be in favor of a no-suction policy, while Holbek et al., in their randomized controlled trial of 2018 (47) compared water seal to a low suction (−10 cmH2O), encountering a significantly shorter chest drain duration in the second group. Finally, Mitsui and coauthors highlighted, in their retrospective report of 2021 (48), as a low-pressure suction (−10 cmH2O) promptly improve postoperative air leaks if compared to lower negative pressures (−20 cmH2O).
Digitally monitored thoracic drainage systems (DDs) are nowadays increasingly used thanks to the possibility of monitoring daily and hourly the air flow and of applying a constant negative pressure avoiding the wall suction, to allow patients’ mobilization (54). DDs effectiveness in PAL patients’ management was evaluated in two prospective studies, compared to traditional (analogue) drainages (TDs). Filosso et al., in 2015 (49), published significantly better results in the group of patients managed with DDs in terms of chest tube duration and length of hospital stay. The Authors concluded that these outcomes may be related to the fact that DDs reduce the interobserver variability on air leak quantification and allow early patient mobilization. In the same year, a RCT from Gilbert et al. showed similar LOS and chest drain removal timing between patients with POAL, randomized to DDs or TDs. The only significant difference was recorded in terms of chest tube clamping trials rate, lower in the DDs group. They hypothesized that DDs can be perceived as more reliable than TDs, and let surgeons remove the tube(s) more confidently. Moreover, only few patients in the TDs group needed the reinsertion of the chest tube after its removal because of PNX and/or subcutaneous emphysema occurrence (50). More recently, Mendogni and colleagues published an interim analysis of preliminary data of a prospective randomized multicentric study which aims to compare DDs to TDs. In contrast with Filosso’s findings, they did not encounter any difference between the two groups, neither in terms of chest drain maintenance or hospitalization, nor in terms of incidence of PAL. The Authors concluded that the only predictor of PAL is the presence of air leak in 1st postoperative day (51).
For medically healed patients with postoperative PAL and with low fluid daily output, a valid option meeting the criteria of Fast-Track protocols can be the discharge with the indwelling chest tube connected to a one-way valve and a collection system. For instance, Varela et al. performed a review on this topic’s literature reporting the experience of four Centers with different devices. Readmission rates varied from 2.2% and 8.3%, mostly because of pleural empyema, and the mean duration of outpatient chest tube management ranged between 7.8 and 11.5 days. They concluded that ambulatory chest drainage after lung resection is a safe procedure in selected patients, and the choice of the device depends on each Center criteria (55).
Moreover, Royer et al., in 2015, described this method as effective in reducing LOS, with a re-admission rate of 3% and nil 30-days mortality on a cohort of 65 patients (56). One year later, Schmocker and colleagues reported their results in patients underwent pulmonary lobectomy/bilobectomy and early discharge protocol, with substantially similar rates of readmission and complications than patients discharged without chest drain. Moreover, they performed a costs analysis which showed a saving of about 686$ per patient (57).
Unfortunately, these studies lack a comparable control group and data on the exact duration of air leak, since the investigators didn’t evaluate patients every day. For these reasons, their results are not suitable for being included in this systematic review.
Discussion
APF and the consequent air leak is the most common complication following lung surgery with an incidence that is reported to be up to 70% intraoperatively and up to 25% after the fifth post-operative day. PAL is the leading cause of postoperative pulmonary morbidity, prolonged hospitalization, and increased hospital costs. In the last decades, surgeons paid close attention to the peri-operative management of the patients underwent surgery for lung cancer, with the double aim of reducing the cost of the hospitalization and enhancing the post-operative recovery. In this scenario, different techniques were tested to reduce clinical and socio-economic impact of the most common post-operative complications. Thus, several studies have been performed on the various surgical and post-operative measures to manage or prevent air leaks following lung resection; most of them concluded that the adopted technique is superior to the standard of care to prevent and handle this complication.
Each study included in this systematic review focuses on a specific surgical technique, agent or strategy to deal with APF management. The studies’ endpoints are well defined and it has been possible for us to achieve data on PAL duration and/or incidence, chest drain removal timing and LOS in the analyzed groups. By contrary, the populations are mostly heterogeneous and for this reason it has not been possible drawing a conclusion on the effectiveness of the single examined strategy by stratifying patients for PAL risk factors (i.e., COPD, emphysema, smoking habit) or for type of surgery (lobectomy/segmentectomy/wedge resection). However, most Authors compared these features between the study and control groups, showing basically no differences; this may mean that those variables have not influenced the results. Authors as Miller (10) and Deguchi (11) highlighted the role of the reinforced staplers that showed a PAL-preventive role, especially in patients with emphysematous lungs. Many authors (12-26), instead, reported their experience with sealants, which number is steadily on the rise, that showed effective and safe sealant capacities. However, due to their costs, availability, and the lack of evidence-based medicine on their routinary use, sealants are, to date, not recommended for PAL prevention but only for intraoperative air leak treatment according to the surgeon’s experience.
Surgical techniques, as fat pads apposition, fissure-less lobectomy, and pleural tenting seems to be safe and effective in preventing PAL but are time-consuming and highly depending on the surgeon’s experience and choices, even if the selected studies did not report any procedure-related complications (27-36).
However, air leaks from APF may not be detected during surgery, and it’s not always possible for surgeons to adopt PAL-preventing strategies intraoperatively. For this reason, most of literature is about postoperative PAL conservative management, that can rely on fewer options than intraoperative air leak treatment. To date, in case of completely expansible residual lung parenchyma, PAL treatment may be safely attempted by autologous blood patch or chemical pleurodesis (37-42), whereas in case of residual pleural space, applying suction to the chest drain is still the most effective option available (43-48). In all cases, digital drainage systems are useful since they allow early patients’ mobilization and a real time quantification of air flow (49-51).
One of the most unsolved issues is the definition of the standard of care, since it is different from center to center. No one of the above-mentioned measures, in fact, has succeeded in becoming the recognized standard of care to prevent or manage PAL, except for the digital drainage system that are increasingly adopted worldwide.
As reported by some authors (6-8), a correct management of prolonged postoperative air leak should begin even before surgery, with the proper identification and, whereas possible, reduction of the risk factors. However, most of them cannot be modified; hence, the adoption of the abovementioned intraoperative precautions, especially in high-risk patients, is advisable. We are far from electing of one of those techniques as “gold standard”, since every equip is more confident with one approach than another and the choice must be tailored on the surgical intervention and on the single patient’s conditions. Even more when all the pre- and intraoperative precaution failed, an accurate post-operative management of the chest drain is essential to handle air leaks and prevent PAL. Measures as autologous blood patch or sclerosant agent to induce a chemical pleurodesis may help to control the PAL; moreover, their cost is not prohibitive although their efficacy is lower than some surgical sealants.
Conclusions
To date, a unanimous consent on the best treatment therapeutic strategy is still far from being achieved. Larger RCTs are needed to better assess which method(s) should deserve to be considered as the standard care of PAL management, but their realization is utopistic because of many hurdles: firstly, the high heterogeneity of patients (especially for what regards the presence of COPD or emphysema), then, the surgeon’s expertise and confidence with each technique, the costs and availability of some agents and sealants and, finally, the absence of a standardized postoperative management of pain control and mobilization.
The increasing diffusion of Enhanced Recovery After Surgery protocols in Thoracic Surgery may help to lay the foundations for studies with more homogeneous baseline population, by improving pre-operative general conditions and promoting standardized measures to reduce post-operative morbidity (including PAL) and hospitalization (58).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Crisci, Alessandro Brunelli, Florian Augustin, Francesco Zaraca) for the series “Prolonged Air Leak after Lung Surgery: Prediction, Prevention and Management” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-736/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-736/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-736/coif). The special series “Prolonged Air Leak after Lung Surgery: Prediction, Prevention and Management” was sponsored by Bard Limited. Bard Limited has no interference on the contents of the special series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis 2014;6:271-84. [Crossref] [PubMed]
- Kozower BD. Complications of thoracic surgical procedures. LoCicero J, Feins RH, Colson YL, et al. editors. Shields’ general thoracic surgery (8th edition). Philadelphia: Lippincott Williams & Wilkins, 2019: 573-585.
- Zaraca F, Pipitone M, Feil B, et al. Predicting a Prolonged Air Leak After Video-Assisted Thoracic Surgery, Is It Really Possible? Semin Thorac Cardiovasc Surg 2021;33:581-92. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Cerfolio RJ, Tummala RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg 1998;66:1726-31. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg 2004;77:1205-10; discussion 1210. [Crossref] [PubMed]
- Jin R, Zheng Y, Gao T, et al. A nomogram for preoperative prediction of prolonged air leak after pulmonary malignancy resection. Transl Lung Cancer Res 2021;10:3616-26. [Crossref] [PubMed]
- Stolz AJ, Schützner J, Lischke R, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:334-6. [Crossref] [PubMed]
- Belda-Sanchís J, Serra-Mitjans M, Iglesias Sentis M, et al. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev 2010;2010:CD003051. [Crossref] [PubMed]
- Miller JI Jr, Landreneau RJ, Wright CE, et al. A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections. Ann Thorac Surg 2001;71:319-22; discussion 323. [Crossref] [PubMed]
- Deguchi H, Tomoyasu M, Shigeeda W, et al. Reduction of air leakage using linear staple device with bioabsorbable polyglycolic acid felt for pulmonary lobectomy. Gen Thorac Cardiovasc Surg 2020;68:266-72. [Crossref] [PubMed]
- Allen MS, Wood DE, Hawkinson RW, et al. Prospective randomized study evaluating a biodegradable polymeric sealant for sealing intraoperative air leaks that occur during pulmonary resection. Ann Thorac Surg 2004;77:1792-801. [Crossref] [PubMed]
- D'Andrilli A, Andreetti C, Ibrahim M, et al. A prospective randomized study to assess the efficacy of a surgical sealant to treat air leaks in lung surgery. Eur J Cardiothorac Surg 2009;35:817-20; discussion 820-1. [Crossref] [PubMed]
- De Leyn P, Muller MR, Oosterhuis JW, et al. Prospective European multicenter randomized trial of PleuraSeal for control of air leaks after elective pulmonary resection. J Thorac Cardiovasc Surg 2011;141:881-7. [Crossref] [PubMed]
- Ueda K, Tanaka T, Li TS, et al. Sutureless pneumostasis using bioabsorbable mesh and glue during major lung resection for cancer: who are the best candidates? J Thorac Cardiovasc Surg 2010;139:600-5. [Crossref] [PubMed]
- Tan C, Utley M, Paschalides C, et al. A prospective randomized controlled study to assess the effectiveness of CoSeal® to seal air leaks in lung surgery. Eur J Cardiothorac Surg 2011;40:304-8. [Crossref] [PubMed]
- Yano T, Haro A, Shikada Y, et al. A unique method for repairing intraoperative pulmonary air leakage with both polyglycolic acid sheets and fibrin glue. World J Surg 2012;36:463-7. [Crossref] [PubMed]
- Lequaglie C, Giudice G, Marasco R, et al. Use of a sealant to prevent prolonged air leaks after lung resection: a prospective randomized study. J Cardiothorac Surg 2012;7:106. [Crossref] [PubMed]
- Klijian A. A novel approach to control air leaks in complex lung surgery: a retrospective review. J Cardiothorac Surg 2012;7:49. [Crossref] [PubMed]
- Petrella F, Borri A, Brambilla D, et al. Efficacy and safety of Innoseal for air leak after pulmonary resection: a case-control study. J Surg Res 2016;206:22-6. [Crossref] [PubMed]
- Gologorsky RC, Alabaster AL, Ashiku SK, et al. Progel Use is Not Associated with Decreased Incidence of Postoperative Air Leak after Nonanatomic Lung Surgery. Perm J 2019;23:18-059. [Crossref] [PubMed]
- Porrello C, Iadicola D, Grutta EM, et al. Routinary use of fibrin sealants to prevent prolonged air leak in thoracic surgery: our experience. G Chir 2019;40:170-3.
- Kawashima M, Kohno T, Fujimori S, et al. Feasibility of autologous fibrin glue in general thoracic surgery. J Thorac Dis 2020;12:484-92. [Crossref] [PubMed]
- Anegg U, Lindenmann J, Matzi V, et al. Efficiency of fleece-bound sealing (TachoSil) of air leaks in lung surgery: a prospective randomised trial. Eur J Cardiothorac Surg 2007;31:198-202. [Crossref] [PubMed]
- Rena O, Papalia E, Mineo TC, et al. Air-leak management after upper lobectomy in patients with fused fissure and chronic obstructive pulmonary disease: a pilot trial comparing sealant and standard treatment. Interact Cardiovasc Thorac Surg 2009;9:973-7. [Crossref] [PubMed]
- Marta GM, Facciolo F, Ladegaard L, et al. Efficacy and safety of TachoSil® versus standard treatment of air leakage after pulmonary lobectomy. Eur J Cardiothorac Surg 2010;38:683-9. [Crossref] [PubMed]
- Yoshimura M, Tsubota N, Matsuoka H, et al. Efficacy of a pedicled pericardial fat pad fixed with fibrin glue on postoperative alveolar air leakage. Surg Today 2002;32:26-8. [Crossref] [PubMed]
- Shintani Y, Inoue M, Funaki S, et al. Clinical usefulness of free subcutaneous fat pad for reduction of intraoperative air leakage during thoracoscopic pulmonary resection in lung cancer cases. Surg Endosc 2015;29:2910-3. [Crossref] [PubMed]
- Stamenovic D, Bostanci K, Messerschmidt A, et al. Fissureless fissure-last video-assisted thoracoscopic lobectomy for all lung lobes: a better alternative to decrease the incidence of prolonged air leak? Eur J Cardiothorac Surg 2016;50:118-23. [Crossref] [PubMed]
- Murakami K, Maehara S, Shimada Y, et al. The Correlation Between Fissureless Technique and Prolonged Air Leak for Patients Undergoing Video-Assisted Right Upper Lobectomy. World J Surg 2021;45:1569-74. [Crossref] [PubMed]
- Brunelli A, Al Refai M, Monteverde M, et al. Pleural tent after upper lobectomy: a randomized study of efficacy and duration of effect. Ann Thorac Surg 2002;74:1958-62. [Crossref] [PubMed]
- Allama AM. Pleural tent for decreasing air leak following upper lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2010;38:674-8. [Crossref] [PubMed]
- Okur E, Kir A, Halezeroglu S, et al. Pleural tenting following upper lobectomies or bilobectomies of the lung to prevent residual air space and prolonged air leak. Eur J Cardiothorac Surg 2001;20:1012-5. [Crossref] [PubMed]
- Marulli G, Droghetti A, Di Chiara F, et al. A prospective randomized trial comparing stapler and laser techniques for interlobar fissure completion during pulmonary lobectomy. Lasers Med Sci 2013;28:505-11. [Crossref] [PubMed]
- Pan XJ, Ou DB, Lin X, et al. Management of Pleural Space After Lung Resection by Cryoneuroablation of Phrenic Nerve: A Randomized Study. Surg Innov 2017;24:240-4. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Shackcloth MJ, Poullis M, Jackson M, et al. Intrapleural instillation of autologous blood in the treatment of prolonged air leak after lobectomy: a prospective randomized controlled trial. Ann Thorac Surg 2006;82:1052-6. [Crossref] [PubMed]
- Andreetti C, Venuta F, Anile M, et al. Pleurodesis with an autologous blood patch to prevent persistent air leaks after lobectomy. J Thorac Cardiovasc Surg 2007;133:759-62. [Crossref] [PubMed]
- Campisi A, Dell'Amore A, Gabryel P, et al. Autologous Blood Patch Pleurodesis: A Large Retrospective Multicenter Cohort Study. Ann Thorac Surg 2022;114:273-9. [Crossref] [PubMed]
- Hasan IS, Allen MS, Cassivi SD, et al. Autologous blood patch pleurodesis for prolonged postoperative air leaks. J Thorac Dis 2021;13:3347-58. [Crossref] [PubMed]
- Jabłoński S, Kordiak J, Wcisło S, et al. Outcome of pleurodesis using different agents in management prolonged air leakage following lung resection. Clin Respir J 2018;12:183-92. [Crossref] [PubMed]
- Chaari Z, Hentati A, Ben Ayed A, et al. Effectiveness and safety of povidone iodine for prolonged lung air-leak after lung surgery. Asian Cardiovasc Thorac Ann 2022;30:314-20. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Comparison of water seal and suction after pulmonary lobectomy: a prospective, randomized trial. Ann Thorac Surg 2004;77:1932-7; discussion 1937. [Crossref] [PubMed]
- Alphonso N, Tan C, Utley M, et al. A prospective randomized controlled trial of suction versus non-suction to the under-water seal drains following lung resection. Eur J Cardiothorac Surg 2005;27:391-4. [Crossref] [PubMed]
- Brunelli A, Sabbatini A, Xiume' F, et al. Alternate suction reduces prolonged air leak after pulmonary lobectomy: a randomized comparison versus water seal. Ann Thorac Surg 2005;80:1052-5. [Crossref] [PubMed]
- Brunelli A, Salati M, Pompili C, et al. Regulated tailored suction vs regulated seal: a prospective randomized trial on air leak duration. Eur J Cardiothorac Surg 2013;43:899-904. [Crossref] [PubMed]
- Holbek BL, Christensen M, Hansen HJ, et al. The effects of low suction on digital drainage devices after lobectomy using video-assisted thoracoscopic surgery: a randomized controlled trial†. Eur J Cardiothorac Surg 2019;55:673-81. [Crossref] [PubMed]
- Mitsui S, Tauchi S, Uchida T, et al. Low suction on digital drainage devices promptly improves post-operative air leaks following lung resection operations: a retrospective study. J Cardiothorac Surg 2021;16:105. [Crossref] [PubMed]
- Filosso PL, Nigra VA, Lanza G, et al. Digital versus traditional air leak evaluation after elective pulmonary resection: a prospective and comparative mono-institutional study. J Thorac Dis 2015;7:1719-24. [Crossref] [PubMed]
- Gilbert S, McGuire AL, Maghera S, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J Thorac Cardiovasc Surg 2015;150:1243-9. [Crossref] [PubMed]
- Mendogni P, Tosi D, Marulli G, et al. Multicenter randomized controlled trial comparing digital and traditional chest drain in a VATS pulmonary lobectomy cohort: interim analysis. J Cardiothorac Surg 2021;16:188. [Crossref] [PubMed]
- Konstantinou F, Potaris K, Syrigos KN, et al. A Novel Technique to Treat Air Leak Following Lobectomy: Intrapleural Infusion of Plasma. Med Sci Monit 2016;22:1258-64. [Crossref] [PubMed]
- Stamenovic D, Messerschmidt A, Steger V, et al. New method in treatment of post-operative air leakage with fresh frozen plasma. ANZ J Surg 2020;90:144-9. [Crossref] [PubMed]
- Dernevik L, Belboul A, Rådberg G. Initial experience with the world's first digital drainage system. The benefits of recording air leaks with graphic representation. Eur J Cardiothorac Surg 2007;31:209-13. [Crossref] [PubMed]
- Varela G, Jiménez MF, Novoa N. Portable chest drainage systems and outpatient chest tube management. Thorac Surg Clin 2010;20:421-6. [Crossref] [PubMed]
- Royer AM, Smith JS, Miller A, et al. Safety of Outpatient Chest Tube Management of Air Leaks After Pulmonary Resection. Am Surg 2015;81:760-3.
- Schmocker RK, Vanness DJ, Macke RA, et al. Outpatient air leak management after lobectomy: a CMS cost analysis. J Surg Res 2016;203:390-7. [Crossref] [PubMed]
- Semenkovich TR, Hudson JL, Subramanian M, et al. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg 2018;30:342-9. [Crossref] [PubMed]