
The value of serum Sema4D level in reflecting the inflammatory state of acute ST-segment elevation myocardial infarction
Highlight box
Key findings
• Our research results showed that changes in sSema4D levels had certain value in reflecting the inflammatory state of STEMI.
What is known and what is new?
• sSema4D promotes thrombosis and may accelerate plaque growth and instability. It is highly expressed in patients with heart failure and atrial fibrillation. CRP is a traditional inflammatory index, which can reflect the inflammatory state of acute myocardial infarction to some extent.
• No previous clinical researches have been conducted on sSema4D levels in patients with STEMI. Now, our study finds that sSema4D levels were not only highly expressed in STEMI group, but also more able to reflect the inflammatory state of STEMI group than CRP.
What is the implication, and what should change now?
• Serum Sema4D level can be used to reflect the inflammatory state of STEMI. The sSema4D levels may have auxiliary value in monitoring the condition of STEMI, which can be expected to become a new inflammatory index for the treatment of STEMI. We should pay more attention to the further study.
Introduction
Acute myocardial infarction (AMI) is a cardiovascular disease caused by myocardial ischemia necrosis, which is caused by the rupture or invasion of coronary atherosclerotic plaque and secondary occlusive thrombosis. It is characterized by an acute onset and high mortality. It is an acute and critical disease of the cardiovascular system.
Studies have proved that smoking, heavy drinking, hypertension, hyperlipidemia and diabetes are risk factors for coronary atherosclerotic heart disease (1-4). Atherosclerosis is not only the result of a large amount of lipid accumulation in the arterial wall, from the formation of lipid striae to the formation of complex atherosclerotic plaque and its complications, but also inflammatory reaction plays a vital role throughout each stage. Such as white blood cell infiltration and the changing expressions of endothelial inflammatory factors.
Semaphorin 4D (Sema4D), also known as CD100, is a homodimer protein that belongs to the signal family of axon guidance proteins. The semaphorin family has become a popular area of research recently due to the multiple functions of these family members in the immune system, which plays an important role in T cell activation, antibody production, and intercellular adhesion (5). The expression of Sema4D usually increases after cell activation as well as under the stimulation of various inflammatory factors (6,7), Sema4D was activated to soluble Sema4D (sSema4D) on the cell surface. sSema4D was expressed by most hematopoietic cells including platelets, neutrophils, T and B lymphocytes, endothelial and monocytes cells. T cells express the highest level of sSema4D, followed by neutrophils, platelets, and monocytes. All these cells are involved in the pathogenesis of atherosclerosis (8,9). sSema4D has been proved to promote the interaction between platelets and platelets, as well as the adhesion of platelets to endothelial and monocytes cells, which is the key step in thrombosis (10).
More and more studies have shown that sSema4D is closely related to the occurrence and development of inflammation, while the study of sSema4D in STEMI is rare.
This study intends to explore the role and potential clinical significance of sSema4D in the occurrence and development of STEMI by detecting the expression of sSema4D in patients with STEMI; The difference of specificity and sensitivity between sSema4D and hs-CRP in reflecting the inflammatory status of patients with STEMI were also studied. We present the following article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-124/rc).
Methods
Subjects
From October 2020 to June 2021, 100 patients with STEMI (56 males and 44 females), 83 with unstable angina (UA) (47 males and 36 females), and 78 (47 males and 31 females) with negative coronary angiography (CAG) who had been admitted to the Department of Cardiology at our hospital were selected and included in this study. The study conforms to the provisions of the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Second Affiliated Hospital of Nantong University (No. 2019KN09), and all subjects signed the informed consent form.
Exclusion criteria and inclusion criteria
In order to be enrolled in this study, subjects must meet the following inclusion criteria: (I) be aged 18–75 years old; (II) meet the diagnostic criteria of the “Guidelines for Primary Diagnosis and Treatment of ST-segment Elevation Myocardial Infarction (2019)” (11); (III) have received a coronary intervention within 12 hours.
Subjects were excluded from the study if they met any of the following exclusion criteria: (I) had severe heart valve disease, severe congenital heart disease, pulmonary heart disease, or hypertrophic obstructive cardiomyopathy; (II) had liver dysfunction [which was defined as alanine transaminase (ALT) or total bilirubin >3 times the upper normal limit], or renal insufficiency (which was defined as serum creatinine >1.5 times the normal upper limit); (III) were at high risk of bleeding; (IV) had an active peptic ulcer or skin ulcer; (V) were allergic to antiplatelet drugs; (VI) had cardiogenic shock; (VII) had a malignant tumor; (VIII) had a left main lesion as shown by CAG.
Treatment methods
All patients were given aspirin (300 mg) and clopidogrel (300 mg) before surgery. After surgery, the patients were given nitrates, aspirin, clopidogrel, atorvastatin, beta blockers, and angiotensin converting enzyme inhibitors according to the “Guidelines for Primary Diagnosis and Treatment of ST-segment Elevation Myocardial Infarction (2019)” (12) to exclude the influence of antiplatelet drugs on the experiment.
Research methods
General information about the patients was collected. Immediately after admission, 2 mL of cubital venous blood was drawn from each patient and placed in a dry lithium heparin blood collection tube and sent to the biochemical laboratory of our hospital for testing. Before surgery, 3 mL of blood was drawn from each patient and placed in dry lithium heparin blood collection tubes and centrifuged at 3,000 ×g for 15 min with a centrifuge. The supernatant was then carefully collected in EP tubes with a pipette. The EP tubes were numbered and grouped, and stored at –80 ℃ in a refrigerator awaiting testing.
All the routine laboratory tests were sent to the biochemical laboratory of our hospital (automatic blood analyzer source: Sysmex Model: XN-9100; automatic biochemical analysis source: Nanjing Sanhe Instrument Co., Ltd.; model: LAboSPECT008AS; Microplate Reader: Thermo Model: Multiskan FC).
Enzyme-linked immunosorbent assays
According to the protocol, the serum Sema4D concentration was measured with enzyme-linked immunosorbent assay kit. First, prepare all kit components and samples at room temperature (18–25 ℃) before use. Establish diluted standard EP tube and blank EP tube. Then add the standard solution or serum sample to the well (100 µL/well) and incubated at 37 ℃ for 2 hours. Remove the liquid from each well, add the working solution of test reagent A and B in turn, and incubate at room temperature for 1 hour. After washing, 3, 3', 5, 5' - tetramethylbenzidine substrate was added to the hole and incubated in the dark for 30 minutes. Finally, the absorbance is measured at 450 nm through a flat reader (Rayto, RT-6500).
Statistical analysis
The data were statistically analyzed using SPSS23.0 medical statistics software. The normally distributed quantitative variables are presented as the mean ± standard deviation. The non-normally distributed quantitative variables are represented by the interquartile P50 (P25, P75). The qualitative variables are represented as frequencies. The normally distributed measurement data were compared using the t-test, the categorical variables were compared using the chi-square test; differences among multiple groups were compared using a 1-way analysis of variance. The non-normally distributed data were tested using a non-parametric test. The receiver operating characteristic (ROC) and area under the curve (AUC) were used to compare the ability of CRP and sSema4D to reflect the inflammatory state of STEMI, and their sensitivity and specificity were calculated. Youden’s index was used to determine the optimal cut-off value. A P value <0.05 was considered statistically significant.
Results
Basic features
There was no significant difference in the levels of clinical indicators, such as age, gender, smoking history, hypertension, diabetes, liver function (ALT), creatinine (Cr), total cholesterol (TC) and Low-Density Lipoprotein Cholesterol (LDL-C) among the groups. The levels of Inflammation index (hs-CRP) (P<0.05), and sSema4D (P<0.001) in STEMI group were higher than those in UA group and control group (Table 1). According to the results, sSema4D can better reflect the inflammatory reaction state than hs-CRP in STEMI group.
Table 1
| Variable | STEMI group (n=100) | UA group (n=83) | Control group (n=78) | P value |
|---|---|---|---|---|
| Age (years) | 65.0 (45.0, 72.0) | 69.0 (56.0, 75.0) | 63.0 (57.0, 70.0) | 0.136 |
| Gender (male), n (%) | 56 (37.3) | 47 (31.3) | 47 (31.3) | 0.835 |
| Smoking, n (%) | 53 (36.6) | 48 (33.1) | 44 (30.3) | 0.794 |
| Hypertension, n (%) | 57 (35.6) | 55 (34.4) | 48 (30.0) | 0.440 |
| Diabetes, n (%) | 29 (46.8) | 19 (30.6) | 14 (22.6) | 0.223 |
| ALT (U/L) | 31.5 (19.0, 49.5) | 28.0 (17.0, 40.0) | 25.0 (19.0, 36.0) | 0.172 |
| Cr (µmol/L) | 65.0 (52.3, 78.4) | 68.2 (59.0, 83.0) | 65.7 (56.4, 80.5) | 0.118 |
| TC (mmol/L) | 4.43±1.34 | 4.18±1.07 | 4.20±1.01 | 0.206 |
| LDL-C (mmol/L) | 2.8 (2.2, 3.5) | 2.7 (2.1, 3.4) | 2.5 (1.9, 3.1) | 0.064 |
| NT-proBNP (pg/mL) | 1,502.5 (698.5, 3,229.5) | 570.0 (102.3, 1,312.0) | 133.5 (57.1, 545.6) | <0.05 |
| hs-CRP (mg/L) | 10.3 (1.4, 35.2) | 3.3 (0.9, 12.3) | 0.5 (0.1, 2.4) | <0.05 |
| sSema4D (ng/mL) | 20.80 (18.52, 23.90) | 17.71 (16.81, 19.76) | 17.70 (16.49, 18.91) | <0.001 |
Data are represented as interquartile P50 (P25, P75) or n (%). sSema4D, soluble semaphorin 4D; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina; ALT, alanine transaminase; Cr, creatinine; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; NT-proBNP, N-terminal precursor B-type brain natriuretic peptide; hs-CRP, high-sensitivity C-reactive protein.
Levels of sSema4D in each group
Compared to the UA group and the control group, hs-CRP and sSema4D in the peripheral blood of the STEMI group were increased, and the differences were statistically significant (hs-CRP: P<0.05; sSema4D: P<0.001) (Figures 1,2). While the hs-CRP and sSema4D levels in UA group and the control group had no statistical significance (P>0.05).
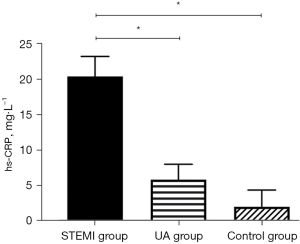
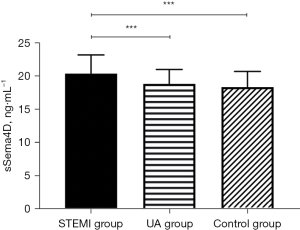
Comparison of hs-CRP and sSema4D in reflecting the inflammatory state of STEMI
The ROC curve was drawn to evaluate the value of hs-CRP (AUC =0.697, cut-off =3.39, 95% CI: 0.629, 0.765, P<0.001) (Figure 3) and sSema4D (AUC =0.780, cut-off =19.62, 95% CI: 0.629, 0.837, P<0.001) (Figure 4) in reflecting the inflammatory state of STEMI. sSema4D is more effective than hs-CRP in reflecting the inflammatory state of STEMI [specificity (sSema4D vs. hs-CRP): 76.4% vs. 66.5%] (Table 2).
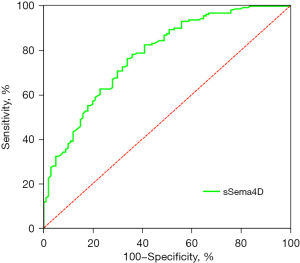
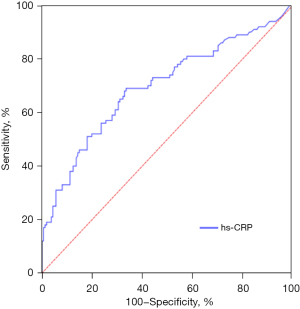
Table 2
| Factor | AUC | 95% CI | P value | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| sSema4D | 0.7800 | 0.629, 0.837 | <0.001 | 19.62 | 66.0 | 76.4 |
| hs-CRP | 0.697 | 0.629, 0.765 | <0.001 | 3.39 | 69.0 | 66.5 |
hs-CRP, high-sensitivity C-reactive protein; sSema4D, soluble semaphorin 4D; STEMI, ST-segment elevation myocardial infarction; AUC, area under the curve.
Discussion
At present, the diagnosis of STEMI mainly depends on troponin I, electrocardiogram, cardiac ultrasound (13), etc. At the same time, many studies have pointed out that STEMI is not only closely related to various acute and chronic inflammation in the process of occurrence and development, but also needed to solve the problem of restenosis caused by inflammatory reaction and neointimal hyperplasia after treatment (14). With the rapid development and popularization of percutaneous coronary intervention (PCI), the restenosis problem after PCI had attracted more and more attention. At present, it was generally recognized that the mechanism is hypoxia or hypoxia in the diseased vascular tissue, repeated balloon dilation and intravascular stent implantation, which cause mechanical injury of vascular intima and local tissue necrosis. The potential inflammatory factors produced can cause acute and chronic inflammatory reaction of vascular wall. Therefore, it is important to find the switch of inflammatory reaction and monitor the occurrence of inflammatory reaction from the source (15).
sSema4D binds to a variety of receptors such as plexin B1/B2, CD72 and plexin C1, which mediate the effects of sSema4D on epithelial cells, immune cells, nerve cells, and endothelial cells, thereby playing a pleiotropic role in immune activation, angiogenesis, bone metabolism, and neurodevelopment (16-20). sSema4D has been confirmed to be expressed in foam cells and plaque macrophages (10). Mou (21) found a dual mechanism for the absence of Sema4D-mediated platelet activation and aggregation. As platelets express both Sema4D and plexinB1, Sema4D, which is initially attached to the surface of platelets, can act as a ligand for platelet-platelet interaction for initial coupling, and then induce platelet activation through the activation of the tyrosine kinase (Syk), thereby promoting thrombosis (22). A previous study has shown that Sema4D mediates the plexinB1 pathway to indirectly promote thrombosis (23). Thrombosis is an important pathological process of AMI.
The underlying mechanism by which sSema4D promotes atherosclerosis is unclear. Studies have shown that it plays a role in promoting cell-to-cell contact in monocyte-endothelial cell interactions, activating platelet responses accelerating angiogenesis and promoting cell-to-cell contact in monocyte-endothelial cell interactions (24-26). Other studies have reported that sSema4D is related to the number of vulnerable atherosclerotic plaques, characterized by increased lipid deposition, accumulation of macrophages and platelets, and increased frequency of arterial occlusion (22,23). Recently, studies have pointed out that the sSEMA4D level in patients with heart failure and atrial fibrillation is increased, but the exact mechanism is unclear (27,28). Can reported that sSema4D seems to be a novel biomarker of the recurrence after the catheter ablation (CA) procedure in paroxysmal atrial fibrillation (PAF) (29). In addition, sSema4D activates platelets, mediates angiogenesis, and accelerates plaque growth and instability (30,31). A previous study showed that sSema4D down-regulated the expression of pro-inflammatory cytokines in macrophages because it weakened the NF of THP-1 macrophages stimulated by lipopolysaccharide-κ activation of B signal pathway (32).
Inflammation is known to be a causative factor in atherosclerosis (33). Chronic inflammation is associated with future cardiovascular events (34). Hs-CRP is a non-specific marker of inflammation and is associated with acute phase responses (35). This study found that sSema4D can reflect the inflammatory state of patients with STEMI, and after comparison, it was found that sSema4D has better specificity than hs-CRP. To sum up, sSema4D is significantly overexpressed in patients with AMI, reflecting the inflammatory status of patients with acute myocardial infarction. Compared with the current non-specific inflammatory indicators, sSema4D is more effective in reflecting the inflammatory status, and may have auxiliary value for monitoring the disease, and may become a new inflammatory indicator for preventing coronary atherosclerosis. More importantly, sSema4D is expected to be a therapeutic target for STEMI, which is worthy of further study.
Conclusions
In conclusion, our study shows that the change of sSema4D level has certain value in evaluating the inflammatory status of STEMI. Therefore, the sSema4D level may be an effective indicator to evaluate the inflammation of STEMI. We intend to expand the sample size and extend the follow-up time to further examine the correlation between the sSema4D level and the inflammatory status of STEMI.
Acknowledgments
The authors acknowledge the support of the Cardiology and Department of General Medicine of The Second Affiliated Hospital of Nantong University. We would also like to thank all the patients and volunteers who participated in this study.
Funding: This work was supported by the Jiangsu Provincial Health Commission Project (grant No. Z2021005, to Koulong Zheng), and Nantong Science and Technology Bureau Plan Project (grant No. MS12020016, to Koulong Zheng).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-124/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-124/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-124/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-124/coif). KZ reports that this work was supported by the Jiangsu Provincial Health Commission Project (grant No. Z2021005), and Nantong Science and Technology Bureau Plan Project (grant No. MS12020016). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of The Second Affiliated Hospital of Nantong University (No. 2019KN09), and all subjects signed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dugan J, Butts A, Collins BSJ, et al. Updated management for patients with cardiovascular disease and diabetes. JAAPA 2019;32:51-3. [Crossref] [PubMed]
- Gallucci G, Tartarone A, Lerose R, et al. Cardiovascular risk of smoking and benefits of smoking cessation. J Thorac Dis 2020;12:3866-76. [Crossref] [PubMed]
- Pedersen TR. The Success Story of LDL Cholesterol Lowering. Circ Res 2016;118:721-31. [Crossref] [PubMed]
- Gong X, Shi J, Huang J, et al. Comparison of Hypertension in Migrant and Local Patients with Atherosclerotic Diseases: A Cross-Sectional Study in Shanghai, China. Ann Glob Health 2020;86:25. [Crossref] [PubMed]
- Maleki KT, Cornillet M, Björkström NK. Soluble SEMA4D/CD100: A novel immunoregulator in infectious and inflammatory diseases. Clin Immunol 2016;163:52-9. [Crossref] [PubMed]
- Nishide M, Nojima S, Ito D, et al. Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis. Ann Rheum Dis 2017;76:1440-8. [Crossref] [PubMed]
- Ha YJ, Han DW, Kim JH, et al. Circulating Semaphorin 4D as a Marker for Predicting Radiographic Progression in Patients with Rheumatoid Arthritis. Dis Markers 2018;2018:2318386. [Crossref] [PubMed]
- Mastenbroek TG, van Geffen JP, Heemskerk JW, et al. Acute and persistent platelet and coagulant activities in atherothrombosis. J Thromb Haemost 2015;13:S272-80. [Crossref] [PubMed]
- Kawano H, Kohno Y, Izumida S, et al. Rivaroxaban therapy resulting in the resolution of right atrial thrombosis resistant to ordinary control with warfarin in a patient with atrial fibrillation. Intern Med 2015;54:601-4. [Crossref] [PubMed]
- Gong H, Lyu X, Li S, et al. sSema4D levels are increased in coronary heart disease and associated with the extent of coronary artery stenosis. Life Sci 2019;219:329-35. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237-69. [Crossref] [PubMed]
- Flather M, Bakhai A, Perez de Arenaza D. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N Engl J Med 2015;373:1274. [Crossref] [PubMed]
- Hui L, Wang D, Liu T, et al. Diagnostic performance of transthoracic echocardiography in screening acute type A aortic dissection from ST-segment elevated myocardial infarction. Cardiovasc Diagn Ther 2022;12:603-13. [Crossref] [PubMed]
- Battisha A, Sawalha K, Madoukh B, et al. Acute Myocardial Infarction in Systemic Mastocytosis: Case Report With Literature Review on the Role of Inflammatory Process in Acute Coronary Syndrome. Curr Cardiol Rev 2020;16:333-7. [Crossref] [PubMed]
- Yuan J, Wang JM, Cai Y, et al. Correlation between ischemic myocardial injury and inflammatory reaction, and anti-inflammatory effect of acupuncture. Zhen Ci Yan Jiu 2019;44:302-6. [Crossref] [PubMed]
- Chen Y, Zhang L, Liu WX, et al. VEGF and SEMA4D have synergistic effects on the promotion of angiogenesis in epithelial ovarian cancer. Cell Mol Biol Lett 2018;23:2. [Crossref] [PubMed]
- Yoshida Y, Ogata A, Kang S, et al. Semaphorin 4D Contributes to Rheumatoid Arthritis by Inducing Inflammatory Cytokine Production: Pathogenic and Therapeutic Implications. Arthritis Rheumatol 2015;67:1481-90. [Crossref] [PubMed]
- Wu JH, Li YN, Chen AQ, et al. Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy. EMBO Mol Med 2020;12:e10154. [Crossref] [PubMed]
- Lontos K, Adamik J, Tsagianni A, et al. The Role of Semaphorin 4D in Bone Remodeling and Cancer Metastasis. Front Endocrinol (Lausanne) 2018;9:322. [Crossref] [PubMed]
- Zuazo-Gaztelu I, Pàez-Ribes M, Carrasco P, et al. Antitumor Effects of Anti-Semaphorin 4D Antibody Unravel a Novel Proinvasive Mechanism of Vascular-Targeting Agents. Cancer Res 2019;79:5328-41. [Crossref] [PubMed]
- Mou P, Zeng Z, Li Q, et al. Identification of a calmodulin-binding domain in Sema4D that regulates its exodomain shedding in platelets. Blood 2013;121:4221-30. [Crossref] [PubMed]
- Zhu L, Stalker TJ, Fong KP, et al. Disruption of SEMA4D ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:1039-45. [Crossref] [PubMed]
- Zhu L, Bergmeier W, Wu J, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A 2007;104:1621-6. [Crossref] [PubMed]
- Luque MC, Gutierrez PS, Debbas V, et al. CD100 and plexins B2 and B1 mediate monocyte-endothelial cell adhesion and might take part in atherogenesis. Mol Immunol 2015;67:559-67. [Crossref] [PubMed]
- Wannemacher KM, Zhu L, Jiang H, et al. Diminished contact-dependent reinforcement of Syk activation underlies impaired thrombus growth in mice lacking Semaphorin 4D. Blood 2010;116:5707-15. [Crossref] [PubMed]
- Conrotto P, Valdembri D, Corso S, et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood 2005;105:4321-9. [Crossref] [PubMed]
- Willner N, Goldberg Y, Schiff E, et al. Semaphorin 4D levels in heart failure patients: a potential novel biomarker of acute heart failure? ESC Heart Fail 2018;5:603-9. [Crossref] [PubMed]
- Xiang L, You T, Chen J, et al. Serum Soluble Semaphorin 4D is Associated with Left Atrial Diameter in Patients with Atrial Fibrillation. Med Sci Monit 2015;21:2912-7. [Crossref] [PubMed]
- Can V, Cakmak HA, Vatansever F, et al. Assessment of the relationship between semaphorin4D level and recurrence after catheter ablation in paroxysmal atrial fibrillation. Biomarkers 2021;26:468-76. [Crossref] [PubMed]
- Yukawa K, Tanaka T, Kishino M, et al. Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med 2010;26:39-44. [Crossref] [PubMed]
- Bawamia B, Mehran R, Qiu W, et al. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J 2013;165:441-50. [Crossref] [PubMed]
- Liu Y, Zhang WS, Tang ZH, et al. Anti-inflammatory effects of the immobilization of SEMA4D on titanium surfaces in an endothelial cell/macrophage indirect coculture model. Biomed Mater 2021;
- Zhang X, Wang S, Fang S, et al. Prognostic Role of High Sensitivity C-Reactive Protein in Patients With Acute Myocardial Infarction. Front Cardiovasc Med 2021;8:659446. [Crossref] [PubMed]
- Arroyo-Espliguero R, Avanzas P, Quiles J, et al. Predictive value of coronary artery stenoses and C-reactive protein levels in patients with stable coronary artery disease. Atherosclerosis 2009;204:239-43. [Crossref] [PubMed]
- Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol 2013;168:5126-34. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


