
One hundred cases of primary spontaneous pneumomediastinum: leukocytosis is common, pleural effusions and age over 40 are rare
Highlight box
Key findings
• Tachycardia and leukocytosis are common in primary spontaneous pneumomediastinum.
• Pleural effusions and age over 40 years are rare in primary spontaneous pneumomediastinum.
What is known and what is new?
• Primary spontaneous pneumomediastinum is a benign condition that can be difficult to discriminate from Boerhaave syndrome due to a shared constellation of history, signs, and symptoms combined with a poor understanding of basic vital signs, labs, and diagnostic findings.
• We provide robust data on vital signs, laboratory values and imaging findings of primary spontaneous pneumomediastinum in 100 patients.
What is the implication, and what should change now?
• Esophagram is not indicated in patients under age 40 without a history of retching or emesis if they have risk factors or history consistent with primary spontaneous pneumomediastinum (e.g., asthma, smoking, Valsalva).
• Fever, pleural effusion, age over 40 and a history of retching or emesis raise concern for esophageal perforation.
Introduction
Primary spontaneous pneumomediastinum (PSPM) is a benign condition that is problematic because it can be confused with Boerhaave syndrome (esophageal rupture). Hamman first described PSPM in 1939 (1). Macklin and Macklin elucidated the pathophysiologic mechanism, showing that sudden intrathoracic pressure changes can cause alveolar rupture and subsequent air tracking in the broncho-vascular tissue plane (2). Consistent with this mechanism, PSPM patients often have a history of cough, asthma, retching, emesis, smoking, or strenuous physical activity (3). The patients are typically young (in their twenties), healthy, and most frequently present with chest pain or dyspnea (4). They may have subcutaneous emphysema and/or a “Hamman sign”, an auscultatory finding of mediastinal crunching synchronous with cardiac contraction (5-13). They may have a history of forced Valsalva events, such as childbirth or cannabinoid hyperemesis syndrome (14,15). However, some present with no identifiable predisposing history or inciting event (8,16).
Why are PSPM patients so difficult to discriminate from patients with esophageal perforation? One important reason is that both conditions frequently share a common constellation of history, symptoms, and signs: chest pain, subcutaneous emphysema, and a recent history of retching or emesis. Additionally, there is a very limited understanding of the vital signs and laboratory values of patients presenting with PSPM. For example, only 3 of 19 spontaneous pneumomediastinum case series commented on the presence of tachycardia (8,9,17). Finally, aside from rare cases where a perforation can be clearly identified on computed tomography (CT), no chest radiographic (CXR or CT) findings are known that can definitively discriminate PSPM from Boerhaave syndrome. We suspected that these factors drive frequent inter-hospital transfers to facilitate access to thoracic surgery consultation or urgent esophagrams. Our aims were to improve our understanding of the clinical presentation, diagnostic evaluation, and management of patients with a descriptive study of PSPM by evaluating a large case series treated within our hospital system. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1136/rc).
Methods
This retrospective case series utilized our Radiology Department’s database to generate a list of all CXR and CT chest studies reporting on “pneumomediastinum” in patients 18 years of age or older between March 2001 and November 2019 at University of Wisconsin (UW) Health. Data were from UW Hospital (a 505-bed regional referral center), UW Health East Madison Hospital (a 55-bed community based hospital and Emergency Department), and Unity Point Health-Meriter (a-448 bed community based hospital) with approximately 70,000 annual inpatient admissions and 200,000 Emergency Department visits. Our project was part of a quality improvement initiative for PSPM patient management at our institution. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board (IRB) approval was deemed unnecessary by institutional review board of the University of Wisconsin Health and consent was waived for this retrospective analysis. The initial search generated 13,286 reports. The vast majority of these commented on the absence of pneumomediastinum. For example, phrases such as “no pneumomediastinum” and “no pneumothorax or pneumomediastinum” were common. Other reports had a positive finding of pneumomediastinum clearly attributable to a specific cause (e.g., postoperative, traumatic, etc.). We selected cases where the etiology of pneumomediastinum was specified as spontaneous or was unclear from the reports. Detailed chart reviews were performed (CTM and EEL), resulting in the identification of 100 cases of PSPM. Predetermined clinical variables were selected from our recent review of nineteen PSPM case series and included demographics, histories, symptoms, clinical signs, and vital signs (3). Vital sign definitions were as follows: fever [defined as a temperature of 38 degrees Celsius (℃) or higher], tachycardia [defined as a heart rate of 100 beats per minute (bpm) or greater], hypotension (defined as systolic blood pressure of less than 90 mmHg), tachypnea (defined as a respiratory rate over 20 per minute), and hypoxia [defined as an oxygen saturation (SpO2) of less than 90%]. Additional clinical variables included laboratory values: white blood cell count (WBC, cells per 109/L), leukocytosis (defined as WBC greater than 11×109/L), hemoglobin (Hgb, grams per deciliter), platelet count (PLT, cells per 109/L), and serum creatinine (Cr, milligram per deciliter). Finally, we evaluated diagnostic studies, management, and treatment approaches, thoracostomy tube placement, hospital admission, length of stay (LOS), and antibiotic use.
Statistical analysis
Descriptive statistical analysis was performed in Excel.
Results
Demographics
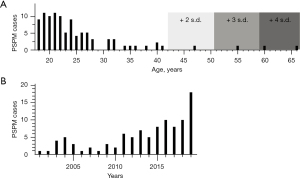
Exactly 100 patients with PSPM were identified between March 2001 and November 2019. Patients ages ranged 18–66 years old (Table 1). The mean age was 25 years. Only three patients were over the age of 50, and only five were over the age of 40 (Figure 1A). There was a male predominance (n=70, 70%) (Table 1). The annual average number of PSPM cases in our system was 5.3, range, 1–18 (Figure 1B).
Table 1
| Demographics | Values |
|---|---|
| Age, range (years) | 18–66 |
| Age, mean ± SD (years) | 25.03±8.5 |
| Male, % | 70 |
| History, % | |
| Cough | 34 |
| Asthma | 27 |
| Retching or emesis | 24 |
| Smoking | 11 |
| Exertional physical activity | 11 |
| Symptoms, % | |
| Chest pain | 76 |
| Dyspnea | 57 |
| Neck pain | 47 |
| Signs, % | |
| Subcutaneous emphysema | 33 |
| Hamman sign | 10 |
| Pneumothorax | 1 |
| Pseudo-pneumothorax | 4 |
SD, standard deviation.
Histories
We evaluated histories reported by the patients. A recent cough (34%) was reported most frequently (Table 1). 27% of the patients reported a history of asthma. Retching or emesis were noted in 24% of the patients. A history of smoking was reported in 11% of the patients. Recent strenuous (e.g., sports) physical activity was reported in 11%. Consistent with a reported recurrence rate of 0.98%, one patient (1%) had a prior PSPM (3). We also found some less common past medical histories (e.g., hyperemesis gravidarum, seizure, diabetic ketoacidosis, overdose on methylphenidate). Many of these have been reported previously but were not common enough to be included in our pre-determined variables.
Symptoms and signs
We next evaluated symptoms and signs reported by our patients. Chest pain (76%) was the most common symptom (Table 1). Dyspnea (57%) was the second most common symptom (Table 1). Finally, neck pain was reported in 47% (Table 1). Subcutaneous emphysema (33%) was the most common sign (Table 1). The presence or absence of a Hamman sign was reported in 13 of the patients in our case series, with ten of those positive for a Hamman sign (Table 1). Five of the patients had imaging reads with “trace” or “tiny” pneumothorax. However, on retrospective review, four had “pseudopneumothorax” with a collection of gas superficial to the parietal pleura rather than being intra-thoracic (Figure S1A,S1B). Only one patient had a true pneumothorax.
Vital signs and laboratory values
We evaluated the vital signs and laboratory values of patients upon presentation. Interestingly, no patient presented with fever (Table 2). Tachycardia was documented in 31% of patients (Table 2). No patients presented with hypotension (Table 2). The mean systolic, diastolic, and mean arterial blood pressure (MAP) at presentation were normal (Table S1). Tachypnea was found in 12% of the patients, but none had hypoxia (Table 2). Leukocytosis was present in 30% of the patients (Table 2). Mean laboratory values at presentation for hemoglobin (14.6), platelet count [346], and creatinine (0.95) were normal (Table S1).
Table 2
| Vital signs and laboratory data | Percent (%) |
|---|---|
| Fever (>38 ℃) | 0 |
| Tachycardia (>100 bpm) | 31 |
| Hypotension (systolic blood pressure <90 mmHg) | 0 |
| Tachypnea (>20 breaths per minute) | 12 |
| Hypoxia (oxygen saturation <90%) | 0 |
| Leukocytosis (WBC >11) | 30 |
Bpm, beats per minute; WBC, white blood cell count (cells per 109/L).
Imaging
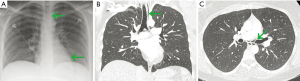
A diagnosis of pneumomediastinum was made by CXR in 94% of patients. However, 66 patients underwent CT of the chest, and 64 patients underwent CT after CXR (Table 3). Despite a concerted review of the imaging reports and notes, we could not reliably determine the exact indications for most of the CT scans performed after CXR identified pneumomediastinum. In two cases, the reason for subsequent CT was to evaluate for pulmonary embolus. In six cases, the diagnosis of pneumomediastinum was not made by CXR, but was made on subsequent CT (Table 3). Three of those cases had subtle pneumomediastinum evident on our retrospective review of the initial CXR (Figure 2A-2C). In two other cases, chest CT was the first imaging obtained and the diagnosis was established by CT. We previously reported no pleural effusion in three earlier case series that included 104 patients (3,18-20). We reviewed both CXR and CT images and reports for pleural effusion in this series. Only one patient was reported to have small pleural effusions on CXR, but no effusion was found on our retrospective review of the CXR (Figure S2). No pleural effusion was found in the 66 patients who did undergo chest CT (Table 3).
Table 3
| Study | Percent [N] |
|---|---|
| PSPM diagnosed by CXR | 94% [98] |
| CT performed | 66% [100] |
| CXR negative, CT positive PSPM | 6% [98] |
| Effusions on CXR | 0% [98] |
| Effusions on CT | 0% [66] |
| Esophagram | 44% [100] |
| EGD | 0% [100] |
| Bronchoscopy | 5% [100] |
PSPM, primary spontaneous pneumomediastinum; CXR, chest X-ray; CT, computed tomography; EGD, esophagogastroduodenoscopy.
Esophagography and other diagnostic procedures
Previously, we found that esophagrams are performed on approximately 36% of PSPM patients, presumably because of concern for esophageal perforation (3). In this study, 44 patients (44%) underwent fluoroscopic esophagography (Table 3). Twenty-four patients (24%) reported a history of retching or emesis. Of these, 16 (67%) underwent esophagography. Surprisingly, twenty-eight (28%) of patients in our series underwent esophagography without a history of retching or emesis. None of our patients underwent an esophagogastroduodenoscopy (EGD) and only five (5%) underwent bronchoscopy (Table 3).
Hospital transfers
Based on our own anecdotal experience, we suspected a high rate of hospital transfers for patients with PSPM due to concern for esophageal perforation. Frequently this is due to the inability to perform, or interpret, esophagography at the referring institution (JD Maloney, personal communication 2018). To our knowledge, there are no reported data regarding transfer rates for PSPM. We found twenty-seven (27%) patients who were transferred from referring facilities; either to our emergency department for triage or as direct admissions (Table 4). Of the nineteen transfers where a reason for transfer could clearly be identified, fifteen (79%) were for concern for esophageal perforation.
Table 4
| Management | Percent [N] |
|---|---|
| Transfers | 27% [100] |
| Transfer for perforation concern | 79% [27] |
| Thoracostomy | 0% [100] |
| Antibiotics | 25% [100] |
| Admission | 57% [100] |
| LOS (days), mean ± SD | 2.3±1 [57] |
PSPM, primary spontaneous pneumomediastinum; LOS, length of stay; SD, standard deviation.
Admissions and management
In this series, the admission rate was 57% with a mean LOS of 2.3±1 days (Table 4). No patients required pleural drain placement for pneumothorax (Table 4). Antibiotics were administered in 25% of patients (Table 4). Due to limitations of the electronic medical record and the retrospective nature of our study, we were unable to determine the duration or durations of the antibiotic regimens. Taken together, these results further demonstrate the resource-intensive care dedicated to PSPM patients.
Discussion
PSPM can be difficult to distinguish from spontaneous esophageal perforation (Boerhaave syndrome) due to a shared constellation of history, signs, and symptoms combined with a poor understanding of basic vital signs, laboratory values, and imaging findings of PSPM. Although not well quantified previously, this diagnostic uncertainty leads to extensive resource utilization to exclude esophageal perforation. Most of our understanding of PSPM comes from small case series (average 28 patients), with 47 patients in the largest previous report (3,21). This study includes 100 PSPM patients and has the expected limitations associated with retrospective studies. Limitations include the retrospective study design with possible selection bias as well as potential for variable definitions of clinical parameters on the patient and electronic medical record levels. Our data may also be confounded by the fact that some patients presented directly to our institution whereas others were transferred after varying durations from presentation and possible interventions at other institutions. In addition, capture of transfer utilization and indications are also limited by the reporting at the electronic medical record level.
Our study demographics correlate well with prior studies (3,4). Notably, we establish that age over 40 was rare. This is an important finding because in a systematic review of 33 case series including 1,452 esophageal perforation patients, Hasimoto et al. found a mean age at presentation of 55.2 years (22). While Boerhaave syndrome cannot be excluded based on age alone, age is clearly a key variable that differs between these two patient populations.
This study cohort provides the first robust data set inclusive of presenting vital signs and laboratory values of PSPM patients. Nearly one-third of patients in our series had tachycardia. We conclude that tachycardia is common in PSPM patients. The absence of fever in our cohort differs from six prior series that had an average rate of 18.8%±10.3% (8,11-13,19,23). However, one study defined fever as a temperature over 38 ℃, one as a temperature over 37.2 ℃, and no definition was reported in four studies. We conclude that the incidence of fever in PSPM needs further study, but is likely uncommon. The prevalence of leukocytosis in our series (31%) is similar to that reported in seven prior case series (30.8%), further validating leukocytosis as a common finding in PSPM cases (8,9,11,16,18,19). We conclude that tachycardia and leukocytosis remain important clinical parameters to consider in patients with possible esophageal rupture, but they are also common in PSPM patients.
Both CXR and CT are adequate to diagnose pneumomediastinum, but they are not able to adequately discriminate between PSPM and esophageal perforation (3). We now know that pleural effusions are rare in PSPM (3,18,19,20). By contrast, ~10–30% of Boerhaave syndrome patients have pleural effusions (20,22). These results greatly expand our understanding of imaging findings of PSPM patients. We conclude that pleural effusions are rare in PSPM and should raise concern for esophageal perforation in patients with pneumomediastinum and a history of retching or emesis.
When suspicion of esophageal perforation is high, esophagography remains the reference standard for diagnosis of esophageal perforation (24). Our results confirm that nearly half of all PSPM patients undergo barium esophagram to rule out esophageal perforation. Unfortunately, in our experience, many centers are unable to perform fluoroscopic esophagram urgently due to staffing or facility limitations. A growing body of evidence has shown that a contrast CT esophagram has a sensitivity and negative predictive value that is equivalent to fluoroscopic esophagram in patients with esophageal perforation (25). This modality should be considered in the workup of patients with pneumomediastinum and a history of retching, emesis, or both. Our finding that 27% of esophagrams were performed on patients with no history of retching or emesis highlights the question of which patients should undergo esophagram. Our results also show that concern for esophageal perforation drives nearly all inter-hospital transfers of PSPM patients.
Finally, our results provide a clearer view of the high level of resource utilization dedicated to PSPM. This included frequent inter-hospital transfers and diagnostic testing due to concern for esophageal perforation in PSPM patients. Most of these patients are admitted for multiple days, and many of them are treated with antibiotics.
Conclusions
PSPM patients frequently present in their twenties with chest pain, tachycardia, subcutaneous emphysema, and leukocytosis. Approximately 25% of PSPM patients have a history of retching or emesis; it is these patients that must be distinguished from those with Boerhaave syndrome. Esophagram is rarely indicated and observation is appropriate in patients under age 40 with a known precipitating event or risk factors for PSPM (e.g., asthma, smoking) if they have no history of retching, emesis, or esophageal disease. Fever, pleural effusion, and age over 40 are rare with PSPM and should raise concern for esophageal perforation in a patient with a history of retching or emesis. More frequent use of CT esophagography in patients where concern for esophageal rupture persists may reassure the evaluating providers and decrease unnecessary patient transfers.
Acknowledgments
The authors thank Dr. Shari Meyerson for critical reading of the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1136/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1136/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1136/coif). DPM has received financial remuneration for consulting services from Atricure and Cook Medical, and for advisory board participation from Lung Bioengineering and Atricure. DPM has grant funding from Ethicon. None of these conflicts have any relevance to the content of this manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board (IRB) approval was deemed unnecessary by institutional review board of the University of Wisconsin Health and consent was waived for this retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hamman L. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hosp 1939;64:1-21.
- Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine (Baltimore) 1944;23:281-358. [Crossref]
- Morgan CT, Maloney JD, Decamp MM, et al. A narrative review of primary spontaneous pneumomediastinum: a poorly understood and resource-intensive problem. J Thorac Dis 2021;13:3721-30. [Crossref] [PubMed]
- Sahni S, Verma S, Grullon J, et al. Spontaneous pneumomediastinum: time for consensus. N Am J Med Sci 2013;5:460-4. [Crossref] [PubMed]
- Abolnik I, Lossos IS, Breuer R. Spontaneous pneumomediastinum. A report of 25 cases. Chest 1991;100:93-5. [Crossref] [PubMed]
- Kaneki T, Kubo K, Kawashima A, et al. Spontaneous pneumomediastinum in 33 patients: yield of chest computed tomography for the diagnosis of the mild type. Respiration 2000;67:408-11. [Crossref] [PubMed]
- Gerazounis M, Athanassiadi K, Kalantzi N, et al. Spontaneous pneumomediastinum: a rare benign entity. J Thorac Cardiovasc Surg 2003;126:774-6. [Crossref] [PubMed]
- Weissberg D, Weissberg D. Spontaneous mediastinal emphysema. Eur J Cardiothorac Surg 2004;26:885-8. [Crossref] [PubMed]
- Campillo-Soto A, Coll-Salinas A, Soria-Aledo V, et al. Spontaneous pneumomediastinum: descriptive study of our experience with 36 cases. Arch Bronconeumol 2005;41:528-31. [Crossref] [PubMed]
- Newcomb AE, Clarke CP. Spontaneous pneumomediastinum: a benign curiosity or a significant problem? Chest 2005;128:3298-302. [Crossref] [PubMed]
- Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110-4. [Crossref] [PubMed]
- Mondello B, Pavia R, Ruggeri P, et al. Spontaneous pneumomediastinum: experience in 18 adult patients. Lung 2007;185:9-14. [Crossref] [PubMed]
- Okada M, Adachi H, Shibuya Y, et al. Diagnosis and treatment of patients with spontaneous pneumomediastinum. Respir Investig 2014;52:36-40. [Crossref] [PubMed]
- Jakes AD, Kunde K, Banerjee A. Case report: Postpartum pneumomediastinum and subcutaneous emphysema. Obstet Med 2019;12:143-5. [Crossref] [PubMed]
- Davis W, Frye K, Shah D, et al. Cannabinoid Hyperemesis Syndrome Presenting With Spontaneous Pneumomediastinum. Prim Care Companion CNS Disord 2020;22:19l02509.
- Ryoo JY. Clinical analysis of spontaneous pneumomediastinum. Tuberc Respir Dis (Seoul) 2012;73:169-73. [Crossref] [PubMed]
- Jougon JB, Ballester M, Delcambre F, et al. Assessment of spontaneous pneumomediastinum: experience with 12 patients. Ann Thorac Surg 2003;75:1711-4. [Crossref] [PubMed]
- Caceres M, Ali SZ, Braud R, et al. Spontaneous pneumomediastinum: a comparative study and review of the literature. Ann Thorac Surg 2008;86:962-6. [Crossref] [PubMed]
- Al-Mufarrej F, Badar J, Gharagozloo F, et al. Spontaneous pneumomediastinum: diagnostic and therapeutic interventions. J Cardiothorac Surg 2008;3:59. [Crossref] [PubMed]
- Bakhos CT, Pupovac SS, Ata A, et al. Spontaneous pneumomediastinum: an extensive workup is not required. J Am Coll Surg 2014;219:713-7. [Crossref] [PubMed]
- Perna V, Vilà E, Guelbenzu JJ, et al. Pneumomediastinum: is this really a benign entity? When it can be considered as spontaneous? Our experience in 47 adult patients. Eur J Cardiothorac Surg 2010;37:573-5. [Crossref] [PubMed]
- Hasimoto CN, Cataneo C, Eldib R, et al. Efficacy of surgical versus conservative treatment in esophageal perforation: a systematic review of case series studies. Acta Cir Bras 2013;28:266-71. [Crossref] [PubMed]
- Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med 2008;102:1329-34. [Crossref] [PubMed]
- Swanson JO, Levine MS, Redfern RO, et al. Usefulness of high-density barium for detection of leaks after esophagogastrectomy, total gastrectomy, and total laryngectomy. AJR Am J Roentgenol 2003;181:415-20. [Crossref] [PubMed]
- Wei CJ, Levenson RB, Lee KS. Diagnostic Utility of CT and Fluoroscopic Esophagography for Suspected Esophageal Perforation in the Emergency Department. AJR Am J Roentgenol 2020;215:631-8. [Crossref] [PubMed]


