Effectiveness of pulmonary artery catheter monitoring for patients with cardiogenic shock of various causes: a systematic review and meta-analysis
Highlight box
Key findings
• There was no significant association between PAC monitoring and in-hospital mortality among patients managed for cardiogenic shock.
What is known and what is new?
• Invasive hemodynamic monitoring with PAC can be useful in the assessment of changes in cardiac function and hemodynamic status.
• We found that the use of PAC in the management of cardiogenic shock was not associated with lower in-hospital mortality, even though it might be beneficial among patients with cardiogenic shock caused by acute decompensated heart failure but not among patients with cardiogenic shock caused by acute coronary syndrome.
What is the implication, and what should change now?
• It is important to select patients with cardiogenic shock who may benefit from PAC.
Introduction
Cardiogenic shock (CS) is characterized by sustained hypotension and systemic hypoperfusion caused by a primary cardiac pump failure in one or both ventricles (1,2). The morbidity and mortality associated with CS remain significant, necessitating sophisticated management even from the time of diagnosis (3). CS is most often caused by acute myocardial infarction; however, there are several causes of shock, and the rates of CS from non-ischemic cardiomyopathy or cardiac causes other than primary myocardial dysfunction have been increasing (4). Therefore, the management of these types of shock is complicated.
During critical care of patients with circulatory failure, it is important to evaluate cardiac function—either directly or indirectly, respectively, through the monitoring of cardiac output or intracardiac pressure—to understand the underlying pathophysiological mechanisms, make a differential diagnosis, and establish appropriate therapeutic goals (5,6). Invasive hemodynamic monitoring with pulmonary artery catheterization (PAC) can be useful for assessing cardiac function (7). Moreover, PAC can provide continuous measurements of several hemodynamic variables, which can facilitate an accurate diagnosis and guide appropriate interventions. Nevertheless, according to the negative results of previous studies, including the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, the routine use of PAC in the management of acute decompensated heart failure (ADHF) is not recommended (8). However, most such previous studies excluded patients with CS. A recent large cohort study demonstrated a decline in PAC use for CS despite improved outcomes (7). Recent studies have shown that PAC guidance is useful in the management of CS, especially in the contexts of specific subtypes, such as postcardiotomy shock or CS from ADHF (9,10). Meanwhile, other studies have yielded controversial findings associated with CS complicating acute myocardial infarction (11). These conflicting data regarding the role of PAC in the management of CS have warranted an organized synthesis of the existing research findings to identify the CS patient most suitable for PAC monitoring. We performed a systematic review and meta-analysis of PAC studies and compared in-hospital mortality among patients with CS of various causes. We present the following article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1139/rc).
Methods
Article selection
Eligible articles included patient groups diagnosed with ADHF or CS. The shock criteria were those used in the SHOCK (Should We Emergently Revascularized Occluded Coronaries for Cardiogenic Shock) and IABP-SHOCK II (Intraaortic Balloon Pump in Cardiogenic Shock II) trials. Patients were categorized dichotomously according to whether or not they underwent hemodynamic monitoring with PAC. The eligible study designs were observational studies or randomized controlled trials. Study outcomes must have included in-hospital mortality. We excluded nonhuman studies and articles using datasets with patients already included in our systematic review and meta-analysis.
Search strategy
We searched for related articles published from 2008 through January 2022 using keywords, such as “pulmonary artery catheterization” and “cardiogenic shock,” in the MEDLINE, Cochrane CENTRAL, and Embase databases. We screened the articles according to our inclusion criteria. Two reviewers analyzed each article separately and reviewed the full text. Disagreements among reviewers were settled by discussions until consensus was reached, with the assistance of a third-party adjudicator if necessary. Data from meta-analyses were extracted by reviewers using prepiloted data collection forms (Appendix 1).
Risk-of-bias assessment
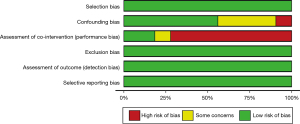
Using the Office of Health Assessment and Translation (OHAT) risk-of-bias method, two reviewers assessed the risk of bias for each article and rated it as either low risk, high risk, or unclear risk of bias according to the following categories: selection bias, confounding bias, assessment of cointervention (performance bias), exclusion bias, detection bias, and selective reporting bias.
Statistical analysis
A random-effects model with an inverse variance method was applied to the analysis to evaluate the effectiveness of PAC for each CS category (according to underlying cause), and Hartung–Knapp adjustment was conducted in the confidence-interval (CI) estimation of treatment effect (12). Chi-square and I2 analyses were conducted to determine statistical heterogeneity assessment, and the Paule-Mandel estimator and Q-profile methods were used for the point and interval estimations of tau2 (13). Publication bias was evaluated using funnel plot. The R package ‘meta’ was used for data analysis in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
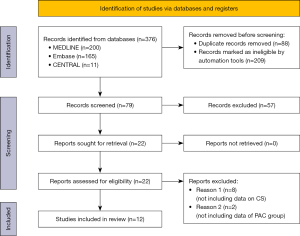
The search strategy flow diagram is shown in Figure 1. Seventy-nine articles were excluded according to the predetermined criteria. Twelve articles were included in the analysis, and approximately 1,854,569 patients met the eligibility criteria. Nine articles reported on retrospective cohort studies, and three reported on prospective cohort studies. No articles reporting on randomized controlled trials met the inclusion criteria. Entire articles were divided according to the cause of CS. Four articles included patients who were close to CS due to acute coronary syndrome (ACS) (14-17), two articles included patients with ADHF without ACS (18,19), and the causes of CS were not accurately described in the other six articles (20-25). The overall baseline characteristics are summarized in Table 1 and Table S1. The mean age of the PAC group was 65.6 years, and there was a male preponderance (66.7%). The inclusion criteria of each study are also summarized in Table 1. In the studies that the cause of CS was not accurately described, the proportions of patients with ACS in the overall cardiogenic shock groups ranged from approximately 40% to 75%. These data were summarized in Table 2.
Table 1
| Study ID | N (%) | Design | Population | Inclusion and exclusion criteria | Definition of CS | Mortality (PAC vs. no PAC) |
|---|---|---|---|---|---|---|
| Ashraf 2020 (14) | 20,758: PAC 1,892 (9.1%), no PAC 18,866 (90.9) | Retrospective cohort, adjusted | National Inpatient Sample (2005–2014) | Inclusion: all patients with AMI-CS who underwent revascularization (PCI or tPA) with MCS | Not provided | 31.18% vs. 28.62% |
| Ha 2018 (15) | 89,718: PAC 5,503 (6.1%), no PAC 84,215 (93.9%) | Retrospective cohort, adjusted | National Inpatient Sample | Inclusion: patients who underwent coronary angiography for the management of AMI complicated by CS | Not provided | 34.9% vs. 29.4% |
| Exclusion: other forms of shock or underwent cardiac surgery, mission information | ||||||
| Vallabhajosyula 2020 (16) | 364,001: PAC 29,609 (8.1%), no PAC 334,392 (91.9%) | Retrospective cohort, adjusted | National inpatient Sample (2000–2014) | Inclusion: patient admitted due to AMI-CS | Not provided | 46.3% vs. 42.0% |
| Exclusion: concomitant cardiac surgery or non-AMI etiology for CS | ||||||
| Oneill 2018 (17) | 13,984: PAC 5,217 (37.3%), no PAC 8,767 (62.7) | Retrospective cohort | Registry that the patient in the US treated with an Impella device (2009–2016) | Inclusion: patient with AMI-CS who were implanted Impella device | Systolic blood pressure <90 mmHg, or need for vasopressor to maintain a systolic blood pressure >90 mmHg | 37% vs. 51% |
| Exclusion: not provided | ||||||
| Sotomi 2014 (18) | 1,004: PAC 502 (50%), no PAC 502 (50%) | Prospective cohort, propensity score matched | ATTEND Registry enrolled patients (2007–2011) | Inclusion: acute heart failure syndrome patients who met the modified Framingham criteria | Not provided | 1.4% vs. 4.4% |
| Exclusion: patients aged <20 years, acute coronary syndrome, defined unsuitable by attending physicians | ||||||
| Rossello 2017 (19) | 65: PAC 43 (66.1%), no PAC 22 (33.9%) | Prospective cohort, adjusted | Single-center registry (2005–2009) | Inclusion: first admission for CS | Not provided | 49% vs. 82% |
| Exclusion: acute coronary syndrome | ||||||
| Doshi 2018 (20) | 842,369: PAC 71,452 (8.5%), no PAC 770,917 (91.5%) | Retrospective cohort, adjusted | National Inpatients sample (2005–2014) | Inclusion: hospitalization with CS | Not provided | 33.9% vs. 38.8% |
| Exclusion: younger than 18 years of age | ||||||
| Hernandez 2019 (21) | 22,278: PAC 11,139 (50%), no PAC 11,139 (50%) | Retrospective cohort, (propensity score matched) | National Inpatient sample (2004–2014) | Inclusion: diagnosis of CS | Not provided | 34.9% vs. 37% |
| Exclusion: <18 years of age, missing mortality data | ||||||
| Ranka 2020 (22) | 269,475: PAC 25,840 (9.6%), no PAC 243,635 (90.4%) | Retrospective cohort, adjusted | Nationwide readmissions Database (2016–2017) | Inclusion: hospitalizations with CS | Not provided | 25.8% vs. 39.5% |
| Exclusion: not provided | ||||||
| Sionis 2020 (23) | 219: PAC 82 (37.4%), no PAC 137 (62.6%) | Prospective cohort | Nine hospitals in 8 European countries (Czech Republic, Denmark, Finland, Greece, Italy, Poland, Portugal, and Spain) (2010–2012) | Inclusion: patients aged >18 years within 6 hours from the identification of CS, with hypotension or severe low output syndrome | Systolic blood pressure <90 mmHg for 30 minutes; need for vasopressor therapy to maintain systolic blood pressure >90 mmHg; symptom and/or signs of systemic and/or pulmonary congestion; symptoms and/or signs of hypoperfusion (altered mental status/confusion, cold periphery, oliguria <0.5 mL/kg/h for the previous 6 hours, blood lactate >2 mmol/L) | 42% vs. 34% |
| Exclusion: shock after cardiac or noncardiac surgery, ongoing hemodynamically significant arrhythmia as the cause of hypotension | ||||||
| Osman 2021 (24) | 124,440: PAC 62,220 (50%), no PAC 62,220 (50%) | Retrospective cohort, adjusted (propensity score matching) | National Inpatient Sample (2015–2018) | Inclusion: patient with CS | Not provided | 24.1% vs. 30.6% |
| Exclusion: patients who underwent only left heart catheterization, missing mortality, age, or sex data, younger than 18 years of age, patients who received concomitant cardiac surgery, transcatheter aortic valve replacement, mitral clipping, or catheter ablation during the same hospitalization; patients who died on the day of admission; patients with the diagnosis of primary pulmonary hypertension; patients who were admitted electively to the hospital; patients who received the IHM after or on the same day of receiving durable LVADs or HT | ||||||
| Sidhu 2017 (25) | 106,258: PAC 7,440 (7%), no PAC 98,818 (93%) | Retrospective cohort, propensity score matching | National Inpatient sample (2010–2014) | Inclusion: diagnosis of CS | Not provided | 30.3% vs. 37.4% |
| Exclusion: not provided |
CS, cardiogenic shock; PAC, pulmonary artery catheterization; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; tPA, tissue plasminogen activator; HT, heart transplantation; LVAD, left ventricular assist devices; MCS, mechanical circulatory support; IHM, invasive hemodynamic monitoring.
Table 2
| Study ID | PAC | No PAC |
|---|---|---|
| Doshi 2018 (20) | ACS: 45.5% | ACS: 53.2% |
| Hernandez 2019 (21) | STEMI 24.3% | |
| Non-STEMI 12.9% | ||
| Acute HF 11.4% | ||
| Ranka 2020 (22) | Unknown | Unknown |
| Sionis 2020 (23) | ACS 74% | ACS 85% |
| Osman 2021 (24) | STEMI 19.9% | STEMI 20.3% |
| Non-STEMI 21.9% | Non-STEMI 22.2% | |
| Sidhu 2017 (25) | Unknown | Unknown |
ACS, acute coronary syndrome; PAC, pulmonary artery catheterization; STEMI, ST-segment elevation myocardial infarction; HF, heart failure.
Risk of bias
Twelve articles were compared and analyzed according to the OHAT risk-of-bias assessment method; they were classified as either low risk, high risk, or unclear according to each category of bias, as shown in Figure 2. Publication bias assessment was performed by funnel plot analysis, which did not reveal significant asymmetries (Figure S1).
Mortality
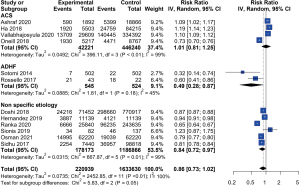
Based on findings from the twelve articles in our meta-analysis, PAC application did not significantly impact in-hospital mortality in the overall CS patient sample [risk ratio (RR) 0.86, 95% CI: 0.73–1.02, I2=100%, P<0.01] (Figure 3). However, two studies investigating CS due to ADHF determined a lower in-hospital mortality in the PAC groups than in the non-PAC groups (RR 0.49, 95% CI: 0.28–0.87, I2=45%, P=0.18). Additionally, six studies investigating CS of unspecified cause observed lower in-hospital mortality in the PAC groups than in the non-PAC groups (RR 0.84, 95% CI: 0.72–0.97, I2=99%, P<0.01). However, there was no significant difference in in-hospital mortality between the PAC and non-PAC groups among patients with CS caused by ACS (RR 1.01, 95% CI: 0.81–1.25, I2=99%, P<0.01).
Quality of evidence
The overall quality of the evidence was deemed to be low because of the high amount of observational data used.
Discussion
In this systematic review and meta-analysis comprising twelve observational studies, the use of PAC did not significantly impact in-hospital mortality among CS patients overall; however, a nonsignificant trend favoring PAC use was found. The results of this review were inconsistent with the results of earlier studies that found PAC to be useful in the management of CS. However, the results of this analysis highlight the need for more evidence based on prospective, randomized clinical trials investigating PAC use in the management of CS.
Since the publication of the ESCAPE trial data in 2005, the use of PAC has decreased in the management of heart failure (7). Interestingly, the use of PAC has also decreased in CS management, even though data regarding its role are insufficient. Most studies investigating PAC monitoring of patients with CS have been retrospective, observational studies with small sample sizes. Several studies have even demonstrated that invasive hemodynamic monitoring with PAC reduces mortality and reduces the need for mechanical circulatory support in a multiplicity of shock contexts (10,18,20). We, therefore, gathered all of the relevant data that we could find and analyzed the role of PAC in CS management. Recently, several meta-analyses have investigated CS-related topics. Chow et al. found that the use of PAC was associated with superior in-hospital mortality outcomes compared with the non-use of PAC (26). In our analysis, the use of PAC did not yield statistically significant results in terms of an association with in-hospital mortality among patients with CS overall. In the meta-analysis conducted by Chow et al., one article reporting on postcardiotomy shock was highly weighted despite its small sample size, and this may have contributed disproportionately to the overall findings and conclusions, pushing the trend toward a more positive effect. Bertaina et al. also conducted a meta-analysis demonstrating the positive role of PAC in the management of CS (27). However, they analyzed only six articles, and there was a possibility of selection bias. To date, the benefit of PAC in CS management remains controversial.
The use of PAC monitoring for patients with CS has decreased even though its use has shown clinical benefits (21). One reason has been the difficulty in interpreting data obtained from PAC studies and the consequent difficulty with clinical decision-making. Another reason has been the poor quality of evidence regarding the use of PAC. Many CS studies have been retrospective, observational studies. Moreover, many of the relevant randomized controlled studies have not proven improvements in outcomes associated with PAC monitoring in the management of various conditions (28,29). It could be argued that sicker patients may receive PAC and have a high a risk of mortality that is not specifically related to the PAC use itself. However, PAC has been reported to cause complications, such as bleeding or infection (8). In this review and meta-analysis, PAC use was not significantly associated with in-hospital mortality among CS patients, even though a nonsignificant trend favoring PAC use was found. This result does not imply that PAC should not be used in the management of CS; rather, the findings may point to an emphasis on better selection of patients who may benefit from PAC.
In the present study, we hypothesized that outcomes associated with invasive hemodynamic monitoring would vary depending on the cause of CS. We found that a specific subgroup of patients with CS caused by ADHF potentially derived a survival benefit from PAC use, while patients with CS caused by ACS did not. Also, the use of PAC did not have a significant benefit in the subgroup of patients with CS cause by non-specific etiology, but the proportion of ACS in these studies was about 40% to 70%. These factors would affect entire analysis. This analysis provides the first evidence of varying PAC-related outcomes according to the underlying cause of shock. Additionally, this is the first meta-analysis to reveal a beneficial effect of PAC for CS caused by ADHF, a result that conflicts with the conventional belief that PAC is not useful in the management of ADHF.
There are several possible explanations for why PAC monitoring was associated with lower in-hospital mortality among patients with CS caused by ADHF but not among patients with CS caused by ACS. In some studies, the hemodynamic profiles of patients with CS caused by ADHF differed from those of patients with CS caused by ACS (10,30). One study found cardiac power output and cardiac index, which are not easily changed, to be key predictors of mortality among patients with CS caused by myocardial infarction, although venous congestion and right-sided heart failure were significant predictors of death in ADHF-CS patients (31,32). Another study demonstrated that right atrial pressure or pulmonary capillary wedge pressure discriminated mortality better in association with CS secondary to heart failure than in association with CS secondary to myocardial infarction (10). These results indicate that invasive hemodynamic monitoring can be more beneficial for patients with CS caused by heart failure because it can be used to monitor hemodynamic profiles in real time and guide decision-making for optimal treatment to improve prognosis. Additionally, the prognosis of CS caused by ACS may be affected by revascularization procedures and interventional outcomes (33). Moreover, the study designs in the included articles may have partly explained the finding of an insignificant benefit of PAC in the management of CS caused by ACS. Most studies used retrospective data, and the proportions of patients using PAC were small overall. This analysis included two articles reporting on CS caused by ADHF without ACS. Although the studies reported in these two articles had relatively small sample sizes, they provided more powerful evidence because they were prospective studies. Additionally, among patients who had CS secondary to ACS, PAC would have been administered to patients with disease of greater severity requiring mechanical circulatory support; this may have contributed to the lack of a significant difference found in the in-hospital mortality between the two groups (16).
There were several limitations to our analysis. First, the quality of evidence was quite low, given that most of the findings were based on observational studies. Second, much of the data were from the National Inpatient Sample. Although this is a large database of patients managed in the United States, even though the studies varied in terms of sampling periods and the presence or absence of adjustments for confounding variables, many studies have overlapping time frames, and there is potential for unmeasured confounding. Caution should, therefore, be taken when interpreting the results. Third, there were only two articles reporting on CS caused by ADHF without ACS, and recent advances in treatment options were not sufficiently reflected in the included studies. Currently, the use of PAC for patients with CS is generally not recommended; however, many studies and meta-analyses have shown that PAC can be useful in specific contexts (according to patient characteristics or the cause of CS, for example). Moreover, because most of the included articles reported on observational studies and lacked high-quality evidence, further prospective studies or randomized controlled trials are warranted to provide strong evidence for these findings.
Conclusions
In this meta-analysis and systematic review of PAC use for patients with CS of various causes, the PAC in monitoring had no significant association with in-hospital mortality. However, in the subgroup of patients with CS without ACS, especially those with CS secondary to ADHF, PAC monitoring may be associated with lower in-hospital mortality. Further research is needed to firmly identify the specific CS patients who may benefit from PAC monitoring.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1139/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1139/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1139/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017;136:e232-68. [Crossref] [PubMed]
- Reyentovich A, Barghash MH, Hochman JS. Management of refractory cardiogenic shock. Nat Rev Cardiol 2016;13:481-92. [Crossref] [PubMed]
- Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [Crossref] [PubMed]
- Berg DD, Bohula EA, van Diepen S, et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes 2019;12:e005618. [Crossref] [PubMed]
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013;369:1726-34. [Crossref] [PubMed]
- Takagi K, Kimmoun A, Sato N, et al. Management of Acute Heart Failure during an Early Phase. Int J Heart Fail 2020;2:91-110. [Crossref] [PubMed]
- De Backer D. Is there a role for invasive hemodynamic monitoring in acute heart failure management? Curr Heart Fail Rep 2015;12:197-204. [Crossref] [PubMed]
- Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294:1625-33. [Crossref] [PubMed]
- Shaw AD, Mythen MG, Shook D, et al. Pulmonary artery catheter use in adult patients undergoing cardiac surgery: a retrospective, cohort study. Perioper Med (Lond) 2018;7:24. [Crossref] [PubMed]
- Garan AR, Kanwar M, Thayer KL, et al. Complete Hemodynamic Profiling With Pulmonary Artery Catheters in Cardiogenic Shock Is Associated With Lower In-Hospital Mortality. JACC Heart Fail 2020;8:903-13. [Crossref] [PubMed]
- Ponamgi SP, Maqsood MH, Sundaragiri PR, et al. Pulmonary artery catheterization in acute myocardial infarction complicated by cardiogenic shock: A review of contemporary literature. World J Cardiol 2021;13:720-32. [Crossref] [PubMed]
- Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 2001;20:3875-89. [Crossref] [PubMed]
- Paule RC, Mandel J. Consensus Values and Weighting Factors. J Res Natl Bur Stand (1977) 1982;87:377-85. [Crossref] [PubMed]
- Ashraf S, Ando T, Adegbala O, et al. Incidence and implications of pulmonary artery catheter use in revascularized patients with myocardial infarction and cardiogenic shock: insights from large us national database. J Am Coll Cardiol 2020;75:912. [Crossref]
- Ha LD, Ogunbayo G, Misumida N, et al. Contemporary outcomes of pulmonary artery catheter use in the management of cardiogenic shock due to acute myocardial infarction. J Am Coll Cardiol 2018;71:A1163. [Crossref]
- Vallabhajosyula S, Shankar A, Patlolla SH, et al. Pulmonary artery catheter use in acute myocardial infarction-cardiogenic shock. ESC Heart Fail 2020;7:1234-45. [Crossref] [PubMed]
- O'Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J 2018;202:33-8. [Crossref] [PubMed]
- Sotomi Y, Sato N, Kajimoto K, et al. Impact of pulmonary artery catheter on outcome in patients with acute heart failure syndromes with hypotension or receiving inotropes: from the ATTEND Registry. Int J Cardiol 2014;172:165-72. [Crossref] [PubMed]
- Rossello X, Vila M, Rivas-Lasarte M, et al. Impact of Pulmonary Artery Catheter Use on Short- and Long-Term Mortality in Patients with Cardiogenic Shock. Cardiology 2017;136:61-9. [Crossref] [PubMed]
- Doshi R, Patel K, Patel P, et al. Trends in the utilization and in-hospital mortality associated with pulmonary artery catheter use for cardiogenic shock hospitalizations. Indian Heart J 2018;70:S496-8. [Crossref] [PubMed]
- Hernandez GA, Lemor A, Blumer V, et al. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure With and Without Cardiogenic Shock. J Card Fail 2019;25:364-71. [Crossref] [PubMed]
- Ranka S, Ioannis M, Tarun D, et al. Right Heart Catheterization/Pulmonary Artery Catheterization Use In Cardiogenic Shock: A Friend Or A Foe? Insights from the Nationwide Readmissions Database. J Card Fail 2020;26:S127. [Crossref]
- Sionis A, Rivas-Lasarte M, Mebazaa A, et al. Current Use and Impact on 30-Day Mortality of Pulmonary Artery Catheter in Cardiogenic Shock Patients: Results From the CardShock Study. J Intensive Care Med 2020;35:1426-33. [Crossref] [PubMed]
- Osman M, Syed M, Patel B, et al. Invasive Hemodynamic Monitoring in Cardiogenic Shock Is Associated With Lower In-Hospital Mortality. J Am Heart Assoc 2021;10:e021808. [Crossref] [PubMed]
- Sidhu G, Samir P, Anurag B, et al. TCT-511 Does Pulmonary Artery Catheterization improve in-hospital outcomes in patients with cardiogenic shock: analysis from National Inpatient Sample (NIS). J Am Coll Cardiol 2017;70:B211. [Crossref]
- Chow JY, Vadakken ME, Whitlock RP, et al. Pulmonary artery catheterization in patients with cardiogenic shock: a systematic review and meta-analysis. Can J Anaesth 2021;68:1611-29. [Crossref] [PubMed]
- Bertaina M, Galluzzo A, Rossello X, et al. Prognostic implications of pulmonary artery catheter monitoring in patients with cardiogenic shock: A systematic review and meta-analysis of observational studies. J Crit Care 2022;69:154024. [Crossref] [PubMed]
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network; Wheeler AP, Bernard GR, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006;354:2213-24.
- Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003;348:5-14. [Crossref] [PubMed]
- Gaubert M, Laine M, Resseguier N, et al. Hemodynamic Profiles of Cardiogenic Shock Depending on Their Etiology. J Clin Med 2020;9:3384. [Crossref] [PubMed]
- Basir MB, Kapur NK, Patel K, et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv 2019;93:1173-83. [Crossref] [PubMed]
- Thayer KL, Zweck E, Ayouty M, et al. Invasive Hemodynamic Assessment and Classification of In-Hospital Mortality Risk Among Patients With Cardiogenic Shock. Circ Heart Fail 2020;13:e007099. [Crossref] [PubMed]
- Bagai J, Brilakis ES. Update in the Management of Acute Coronary Syndrome Patients with Cardiogenic Shock. Curr Cardiol Rep 2019;21:17. [Crossref] [PubMed]