
Identified optimal candidates for pulmonary resection in octogenarians with non-small cell lung cancer: a web-based predictive model
Highlight box
Key findings
• A web-based predicted model was constructed to distinguish specific patients who can indeed benefit from pulmonary resection among octogenarians with non-small cell lung cancer (NSCLC).
What is known and what is new?
• A survival benefit from pulmonary resection was observed in octogenarians with NSCLC;
• A web-based predicted model was constructed to identify patients who can indeed benefit.
What is the implication, and what should change now?
• Identifying optimal candidates for pulmonary resection in octogenarians with NSCLC is crucial for individual therapy strategies and improving their prognosis.
Introduction
With the popularity of low-dose computed tomography (LDCT) as a chest physical examination for the past few years, the detection rate of lung cancer particularly those characterized by early-stage and ground-glass nodules has greatly elevated (1). Meanwhile, the proportion of elderly patients is also growing gradually with the aging of the population (2,3). Approximately 14% of non-small cell lung cancer (NSCLC) patients were older than 80 years old (4,5).
Surgeons are increasingly confronted with the question of how to identify the optimal candidates for pulmonary resection in octogenarians with NSCLC. The American College of Chest Physicians the European Respiratory Society and the European Society of Thoracic Surgeons have recommended that elderly patients who are potential candidates for resection should be evaluated for surgery regardless of age (6,7). Despite this and the increased proportion of localized tumors, the pulmonary resection rate in octogenarians has decreased (5,8,9).
Numerous previous studies showed that NSCLC patients who received pulmonary resection had more postoperative complications, more perioperative mortality, and worse long-term survival in octogenarians and older patients compared with young patients (5,9-13), which has led to a controversy over the appropriateness of subjecting octogenarian patients with newly diagnosed NSCLC to surgical resection. Recently, the differences between surgery and non-surgery only in octogenarians have been investigated in several retrospective studies. Surgical resection of NSCLC is associated with improved long-term survival in a substantial proportion of octogenarians and should not be withheld based on age alone (14-17).
Hitherto the role of surgical resection of NSCLC for patients aged 80 years and above and the specific group of octogenarians who do benefit are still not fully clear. We, therefore, undertook this study to establish a user-friendly web-based clinicopathologic prediction model to identify optimal candidates for surgical resection of NSCLC in octogenarians to give a suggestion for individual therapy strategies and comprehensively understand their characteristics in a large population of patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-997/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethical Committee of Shanghai Cancer Center, Fudan University (IRB No. 2008223-9).
Study cohort
The Surveillance, Epidemiology and End Results (SEER) database (2000–2019, November 2021 submission) is a public database collecting tumor-associated data from 17 population-based cancer registries and accounts for approximately 28% of the population in the United States. Patients aged no less than 80 years diagnosed from 2010 to 2018 were extracted.
The inclusion and exclusion criteria are listed as follows:
Inclusion criteria:
- Lung and bronchus tumor (C34.0–34.9) with histologic types of adenocarcinoma (8140–8141, 8143–8144, 8147, 8190, 8201, 8210–8211, 8221, 8230–8231, 8250–8257, 8260–8263, 8265, 8290, 8310, 8315, 8320, 8323, 8333, 8380–8384, 8401, 8440–8441, 8460, 8470, 8480–8482, 8490, 8504, 8510, 8512, 8514, 8525, 8542, 8550–8551, 8570–8576), squamous cell (8015, 8050–8052, 8070–8073, 8075–8076, 8078, 8082–8084, 8123) and others (8560, 8012, 8014, 8034, 8000–8001, 8003, 8005, 8010–8011, 8020–8021, 8023, 8035, 8040, 8046, 8120, 8146, 8951, 8981, 8200, 8430, 8562, 8982, 8004, 8022, 8030–8033, 8074, 8972, 8980);
- Lung cancer was the only or first primary cancer diagnosis;
- Aged more than or equal to 80 years.
Exclusion criteria:
- Patients whose diagnosis was obtained through death certificate or autopsy;
- Patients with unknown race information, unknown tumor position, unknown histological grade, unknown tumor-node-metastasis (TNM) stage, unknown treatment modality, or incomplete survival time;
- Patients whose information is not enough for the reclassification of TNM stage according to the eighth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging manual;
- Patients whose surgery to primary site record was unknown.
The TNM stage was reclassified according to the eighth edition of the AJCC Cancer Staging manual.
Propensity-score matching (PSM)
The eligible patients were divided into the surgery group and the non-surgery group based on the treatment of the primary site. Specifically, patients who received pulmonary resection (pneumonectomy, lobectomy, segmentectomy, and wedge resection) were classified into the surgery group. Differences in confounding variables between the two were compared through the chi-squared test (categorical variables), t-test, or Wilcoxon rank-sum test (continuous variables). PSM was utilized to eliminate the imbalance of confounding variables between the two groups. Variables that have an impact on the outcomes of treatment were assigned a propensity score through the logistic regression, including age, gender, race, tumor site, histologic type, grade, TNM stage, radiotherapy, and chemotherapy. After PSM with the nearest-neighbor method (caliper =0.0001), patients were matched at a 1:1 ratio. Kaplan-Meier estimate and log-rank test were used to compare the difference in survival between groups.
Construction and validation of the nomogram
Cox proportional hazards regression model and competing risk model were used to identify independent prognostic factors of overall survival (OS) and cancer-specific survival (CSS) in the matched population, respectively.
Patients who received surgical resection of NSCLC in matched population were randomly split into the training cohort and validation cohort at a ratio of 2:1. Meanwhile, patients in the surgery group were classified into the nonbeneficial group (CSS time ≤ median CSS time of the non-surgery group) and the beneficial group (CSS time > median CSS time of the non-surgery group).
A multivariable logistic regression model was established in the training cohort, including variables identified as the independent prognostic factors of OS and CSS or available before surgery.
A nomogram identifying octogenarian patients who can indeed benefit from surgical resection of NSCLC was constructed based on the above-mentioned logistic regression model. Receiver operating characteristic (ROC) curves and calibration plots were utilized to assess the predictive capability of the established nomogram. Furthermore, decision curve analyses (DCA), quantifying the net benefits with different threshold probabilities, were used to evaluate the clinical application of the nomogram.
Risk group stratification
Patients in matched population were calculated the predicted probabilities of benefit from NSCLC surgery-resection based on the established nomogram, and divided into the non-surgery group, surgery & non-beneficial group (predicted probability ≤50%), and surgery & beneficial group (predicted probability >50%). Kaplan-Meier survival curves and the log-rank test were used to illustrate the independent discrimination ability of the established nomogram.
Statistical analyses
All statistical analyses were performed in R software (version 4.1.3) with the R packages as follows: “MatchIt”, “tableone”, “foreign”, “survival”, “plyr”, “cmprsk”, “riskRegression”, “rms”, “survminer”, “forestplot”, “broom”, “rmda”, “pROC”, “DynNom”.
Results
Patient characteristics
A total of 14,264 eligible patients with NSCLC were identified from the SEER database from 2010 to 2018 (Figure 1). Of these eligible patients, 4,475 (31.37%) underwent pulmonary resection. Obvious differences in factors including age, gender, race, tumor site, histologic type, grade, TNM stage, the performance radiotherapy, and chemotherapy indicated the imbalanced baseline characteristics of the two groups (surgery group and non-surgery group) (Table 1). Younger and female patients with White race, lobe-located tumor, adenocarcinoma, grade I–II, stage T1–T2, stage N0–N1, and stage M0 were more likely to receive surgery. Variables that affected the outcomes of treatment were included in PSM, including age, gender, race, tumor site, histologic type, grade, TNM stage, radiotherapy, and chemotherapy.
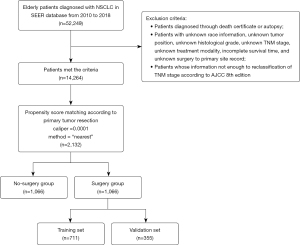
Table 1
| Variables | All patients (n=14,264) | Patients before PSM | Patients after PSM | |||||
|---|---|---|---|---|---|---|---|---|
| Non-surgery (n=9,789) | Surgery (n=4,475) | P | Non-surgery (n=1,066) | Surgery (n=1,066) | P | |||
| Age (years), mean (SD) | 83.67 (3.25) | 84.07 (3.43) | 82.79 (2.60) | <0.001 | 83.39 (2.94) | 83.30 (2.95) | 0.484 | |
| Gender, n (%) | <0.001 | 0.696 | ||||||
| Female | 7,269 (51.0) | 4,831 (49.4) | 2,438 (54.5) | 565 (53.0) | 555 (52.1) | |||
| Male | 6,995 (49.0) | 4,958 (50.6) | 2,037 (45.5) | 501 (47.0) | 511 (47.9) | |||
| Race, n (%) | <0.001 | 0.147 | ||||||
| White | 12,066 (84.6) | 8,169 (83.5) | 3,897 (87.1) | 941 (88.3) | 928 (87.1) | |||
| Black | 890 (6.2) | 707 (7.2) | 183 (4.1) | 53 (5.0) | 44 (4.1) | |||
| Other | 1,308 (9.2) | 913 (9.3) | 395 (8.8) | 72 (6.8) | 94 (8.8) | |||
| Tumor site, n (%) | <0.001 | 0.507 | ||||||
| Lobe | 13,810 (96.8) | 9,389 (95.9) | 4,421 (98.8) | 1,056 (99.1) | 1,057 (99.2) | |||
| Bronchus | 315 (2.2) | 308 (3.1) | 7 (0.2) | 8 (0.8) | 5 (0.5) | |||
| Over lapping lesion | 139 (1.0) | 92 (0.9) | 47 (1.1) | 2 (0.2) | 4 (0.4) | |||
| Laterality, n (%) | 0.272 | 0.723 | ||||||
| Right | 8,257 (57.9) | 5,636 (57.6) | 2,621 (58.6) | 642 (60.2) | 651 (61.1) | |||
| Left | 6,007 (42.1) | 4,153 (42.4) | 1,854 (41.4) | 424 (39.8) | 415 (38.9) | |||
| Histologic type, n (%) | <0.001 | 0.491 | ||||||
| Adenocarcinoma | 8,112 (56.9) | 4,977 (50.8) | 3,135 (70.1) | 619 (58.1) | 636 (59.7) | |||
| Squamous cell | 4,616 (32.4) | 3,548 (36.2) | 1,068 (23.9) | 357 (33.5) | 354 (33.2) | |||
| Other | 1,536 (10.8) | 1,264 (12.9) | 272 (6.1) | 90 (8.4) | 76 (7.1) | |||
| Grade, n (%) | <0.001 | 0.296 | ||||||
| I | 1,840 (12.9) | 1,015 (10.4) | 825 (18.4) | 132 (12.4) | 152 (14.3) | |||
| II | 5,346 (37.5) | 3,228 (33.0) | 2,118 (47.3) | 426 (40.0) | 436 (40.9) | |||
| III–IV | 7,078 (49.6) | 5,546 (56.7) | 1,532 (34.2) | 508 (47.7) | 478 (44.8) | |||
| T, n (%) | <0.001 | 0.719 | ||||||
| T1 | 3,760 (26.4) | 1,932 (19.7) | 1,828 (40.8) | 314 (29.5) | 310 (29.1) | |||
| T2 | 3,976 (27.9) | 2,359 (24.1) | 1,617 (36.1) | 355 (33.3) | 335 (31.4) | |||
| T3 | 3,110 (21.8) | 2,409 (24.6) | 701 (15.7) | 241 (22.6) | 253 (23.7) | |||
| T4 | 3,418 (24.0) | 3,089 (31.6) | 329 (7.4) | 156 (14.6) | 168 (15.8) | |||
| N, n (%) | <0.001 | 0.694 | ||||||
| N0 | 8,310 (58.3) | 4,667 (47.7) | 3,643 (81.4) | 760 (71.3) | 744 (69.8) | |||
| N1 | 1,221 (8.6) | 782 (8.0) | 439 (9.8) | 85 (8.0) | 94 (8.8) | |||
| N2–3 | 4,733 (33.2) | 4,340 (44.3) | 393 (8.8) | 221 (20.7) | 228 (21.4) | |||
| M, n (%) | <0.001 | 0.067 | ||||||
| M0 | 9,856 (69.1) | 5,530 (56.5) | 4,326 (96.7) | 929 (87.1) | 957 (89.8) | |||
| M1 | 4,408 (30.9) | 4,259 (43.5) | 149 (3.3) | 137 (12.9) | 109 (10.2) | |||
| Radiotherapy, n (%) | ||||||||
| Received | 5,500 (38.6) | 5,145 (52.6) | 355 (7.9) | <0.001 | 260 (24.4) | 291 (27.3) | 0.138 | |
| Chemotherapy, n (%) | ||||||||
| Received | 3,023 (21.2) | 2,593 (26.5) | 430 (9.6) | <0.001 | 156 (14.6) | 178 (16.7) | 0.211 | |
PSM, propensity-score matching; SD, standard deviation.
After PSM, 2,132 elderly patients were enrolled in the following analysis. Baseline characteristics including age, gender, race, tumor site, histologic type, grade, TNM stage, radiotherapy, and chemotherapy were all balanced (P>0.05).
Pulmonary resection affects the outcomes of elderly NSCLC patients
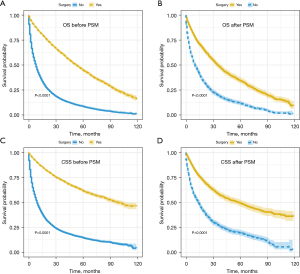
Surgical resection of NSCLC was decreasingly performed with age, especially in patients aged 80 and above (Figure S1). Through the Kaplan-Meier curve and log-rank test, patients who received surgery had an improved OS and CSS than those not (Figure 2). After PSM, the median CSS time of the surgery and non-surgery group was 58 months [95% confidence interval (CI): 48–67 months] and 14 months (95% CI: 12–16 months), respectively. The 1-,2-, and 5-year OS and CSS rates were also calculated (Table S1).
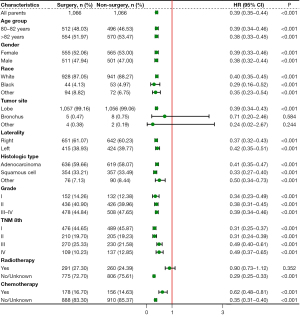
Subgroup analyses of CSS were also performed considering the influences of confounding factors (Figure 3). Pulmonary resection could provide a significant survival benefit regardless of the TNM stage at the time of diagnosis. However, patients with tumors located in the bronchus and others and those who received radiotherapy might not obtain a benefit.
Independent prognostic factors
Independent prognostic factors of OS and CSS were identified through the multivariable Cox regression model and competing risk model, respectively (Table 2). Pulmonary resection was an independent factor associated with improved OS [hazard ratio (HR): 0.39, 95% CI: 0.35–0.43, P<0.001] and CSS (HR: 0.44, 95% CI: 0.39–0.49, P<0.001) in matched population. Furthermore, gender, race, histologic type, grade, TNM stage, radiotherapy, and chemotherapy were confirmed to be independent prognostic factors.
Table 2
| Variables | OS | CSS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Surgery | 0.39 (0.35–0.43) | <0.001 | 0.44 (0.39–0.49) | <0.001 | |
| Age (years) | |||||
| 80–82 | Reference | Reference | |||
| >82 | 1.07 (0.97–1.18) | 0.149 | 0.96 (0.86–1.08) | 0.520 | |
| Gender | |||||
| Female | Reference | Reference | |||
| Male | 1.35 (1.23–1.49) | <0.001 | 1.15 (1.02–1.29) | 0.024 | |
| Race | |||||
| White | Reference | Reference | |||
| Black | 0.94 (0.74–1.18) | 0.570 | 0.92 (0.68–1.23) | 0.560 | |
| Other | 0.82 (0.68–1.00) | 0.047 | 0.94 (0.76–1.15) | 0.540 | |
| Tumor site | |||||
| Lobe | Reference | Reference | |||
| Bronchus | 1.53 (0.86–2.73) | 0.150 | 1.94 (0.97–3.92) | 0.063 | |
| Over lapping lesion | 1.14 (0.47–2.76) | 0.765 | 1.93 (0.84–4.46) | 0.120 | |
| Laterality | |||||
| Right | Reference | Reference | |||
| Left | 0.98 (0.89–1.08) | 0.692 | 0.95 (0.85–1.07) | 0.410 | |
| Histologic type | |||||
| Adenocarcinoma | Reference | Reference | |||
| Squamous cell | 1.14 (1.02–1.28) | 0.018 | 0.98 (0.85–1.12) | 0.710 | |
| Other | 1.41 (1.17–1.69) | <0.001 | 1.17 (0.93–1.48) | 0.180 | |
| Grade | |||||
| I | Reference | Reference | |||
| II | 1.47 (1.25–1.74) | <0.001 | 1.40 (1.14–1.71) | 0.001 | |
| III–IV | 1.66 (1.40–1.97) | <0.001 | 1.83 (1.49–2.25) | <0.001 | |
| T | |||||
| T1 | Reference | Reference | |||
| T2 | 1.26 (1.11–1.42) | <0.001 | 1.58 (1.35–1.83) | <0.001 | |
| T3 | 1.50 (1.31–1.72) | <0.001 | 1.94 (1.64–2.29) | <0.001 | |
| T4 | 1.72 (1.46–2.02) | <0.001 | 2.09 (1.72–2.55) | <0.001 | |
| N | |||||
| N0 | Reference | Reference | |||
| N1 | 1.24 (1.04–1.48) | 0.015 | 1.12 (0.89–1.42) | 0.320 | |
| N2–3 | 1.53 (1.33–1.75) | <0.001 | 1.59 (1.37–1.85) | <0.001 | |
| M | |||||
| M0 | Reference | Reference | |||
| M1 | 1.82 (1.56–2.13) | <0.001 | 1.86 (1.54–2.24) | <0.001 | |
| Radiotherapy | 0.76 (0.68–0.85) | <0.001 | 0.86 (0.75–0.98) | 0.026 | |
| Chemotherapy | 0.66 (0.56–0.77) | <0.001 | 0.77 (0.65–0.91) | 0.002 | |
OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval.
Predicted model to identify optimal candidates for pulmonary resection of NSCLC
The median CSS time of the non-surgery group was 14 months in the matched population. We assumed that patients who received surgical resection of NSCLC and lived longer than 14 months could benefit from the surgery. A total of 750 (70.4%) patients who lived longer than 14 months after surgery were classified into the beneficial group and the others (316, 29.6%) were classified into the non-beneficial group (CSS ≤14 months).
A multivariable logistic regression model was established in the training cohort, in which variables identified as the independent prognostic factors of OS and CSS or available before surgery were included. Although not identified as a survival-affected factor, age was also included considering that older patients had shorter survival.
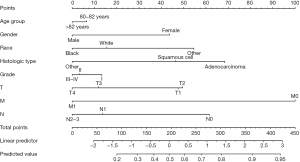
A nomogram, which could identify the optimal surgery candidates from octogenarians with NSCLC, was constructed after a multivariable logistic regression model (Figure 4). TNM stage and histologic type were the leading factors of prognosis, followed by race, gender, grade, and age.
Clinical use
To create user-friendly access, the established nomogram was carried out in a web-based nomogram. Users can estimate the individual probability of benefit from surgery by choosing the clinicopathologic information. Figure 5 shows a screenshot of the web-based nomogram (https://lcss.shinyapps.io/DynNomapp/). For example, a male, white, 83-year-old patient with grade I and T1N0M1 stage lung adenocarcinoma has an 81% probability of benefit from pulmonary resection estimated through our nomogram.
Nomogram validation
ROC curves, calibration plots, and DCA curves were used to evaluate the discrimination and predictive capability of the established nomogram (Figure S2). The areas under the curve (AUCs) of ROC curves were 0.714 and 0.718 in the training cohort and validation cohort, respectively. Calibration plots showed good consistency between the actual observation and estimated probability in both internal and external validation. In DCA, the net benefit of the established nomogram is better than that of the all-treatment or non-treatment scheme when the threshold probability was 0.40 to 0.93.
To validate the distinguishability of the established nomogram, patients in the matched population were classified into the non-surgery group, non-beneficial & surgery group, and beneficial & surgery group based on the nomogram-predicted probability. Through the Kaplan-Meier curve, the beneficial & surgery group has a significantly improved CSS than the non-surgery group (HR: 0.34, 95% CI: 0.30–0.38) and non-beneficial & surgery group (HR: 0.35, 95% CI: 0.28–0.44). Meanwhile, no significant difference in CSS was observed between the latter two groups (P=0.672). similar results of OS analysis were observed (Table S2, Figure S3). We also observed no significant difference in non-cancer-specific survival (NCSS) between the beneficial & surgery group and the non-beneficial & surgery group (P=0.258). Based on the surgical approach, patients were further divided into different subgroups (wedge resection, segmental resection, and lobectomy). Figure S4 shows that, across all subgroups, the beneficial group identified in our study has a better prognosis than those in the non-beneficial group.
Discussion
Appropriate surgical resection of NSCLC in elderly patients especially octogenarians is a crucial issue, several retrospective studies have confirmed pulmonary resection could improve the prognosis of certain patients (18,19). For certain patients 80 years of age or older with early-stage NSCLC who can tolerate lobectomy, Mimae et al. investigated the difference in survival that different surgical approaches might bring about and found that wedge resection may be equivalent to lobectomy or segmentectomy (20), which is in line with Mimae’s previous study (21). Elderly patients, especially octogenarians, were described as being underrepresented in cancer clinical trials for evaluating treatments for NSCLC in a series of articles (22-25). The most recent Japanese prospective randomized clinical trial (JACS1303) has only been conducted on elderly patients to integrate analysis of surgical strategy for elderly lung cancer patients (26), and the current series shows that octogenarians can be successfully treated through surgical resection with an acceptable rate of severe complications and mortality. They retrospectively this data and found that mediastinal lymph node dissection (MLND) had no significant impact on the prognosis of octogenarians with NSCLC (27). In contrast to all previous studies, we only focused on how to identify patients who could indeed benefit from surgical resection of NSCLC to help thoracic surgeons make the best choices. As far as we are aware, our work is the first to develop a web-based, user-friendly prediction model that identifies the optimal candidates for surgical resection of NSCLC based on a large population of octogenarian patients.
Our results revealed several remarkable findings. Firstly, certain octogenarians with NSCLC have been shown that could benefit from surgery. pulmonary resection was associated with improved OS (HR: 0.39, 95% CI: 0.35–0.43) and CSS (HR: 0.44, 95% CI: 0.39–0.49) in octogenarians, in line with prior research. Furthermore, we have developed a web-based, user-friendly predicted nomogram based on a logistic regression model to identify optimal candidates for pulmonary resection in octogenarians and to thorough understanding of their clinical characteristics. Thirdly, the utility and stability of the predicted model were validated by ROC curves, calibration plots, DCA curves, and risk group stratification.
We constructed the predictive model to distinguish optimal candidates for pulmonary resection referred to the design of Zheng et al. and Liang et al. (28,29). Firstly, octogenarians with NSCLC who received pulmonary resection or not were enrolled and matched through PSM in this study. After PSM, patients who underwent surgical resection of NSCLC had a significantly improved CSS than those who did not (median time: 58 vs. 14 months). The surgery group was further split into the beneficial group and non-beneficial group based on the median CSS of the non-surgery group, and their clinicopathological variables were included in a logistic regression model. This model’s precise discriminative capability indicated its generalizability. For the convenience of user access, we modified the model by implementing underlying statistical formulas in web-based nomograms. Finally, patients who could benefit from surgical resection of NSCLC were selected. Based on the surgical approach, patients were further divided into three subgroups (wedge resection, segmental resection, and lobectomy). Among all subgroups, the beneficial & surgery group consistently has an improved survival than those in non-beneficial & surgery group.
In our model, histologic type and TNM stage are the top two strongest factors to predict whether a certain patient could benefit from surgery. As mentioned before, female patients with early TNM-stage and well-differentiated adenocarcinoma had a better prognosis than other octogenarian NSCLC patients. These findings suggested that individual characteristics and the tumor itself had an impact on the outcome of octogenarian patients, possibly as a result of the relatively lower difficulty of the surgical procedures and the prolonged life expectancy.
Several limitations of our study must be taken into account. Firstly, the SEER database lacks data on comorbidities, nutritional status, and performance status (such as cardiac and pulmonary functions), all of which are valuable for surgeons to evaluate the surgical potential and make a clinical decision. CSS was chosen as the primary endpoint, which could somewhat mitigate the effects of missing the aforementioned non-cancer factors. In other words, cancer-specific death excluded the death from non-cancer events, which could help us minimize the bias. The incorporation of it into a more integrated predicted model in future research is crucial. Secondly, the incomplete record of chemotherapy, radiotherapy and immunotherapy could not answer the question that whether underlying treatment would improve the outcome of surgery in this study. Thirdly, this study is retrospective in essence. To identify specific patients who might benefit from surgical resection, prospective randomized clinical trials are still necessary. At all events, this model could be useful for future clinical trial design since it can calculate surgical beneficial probability based on SEER-accessible clinicopathologic characteristics.
Conclusions
A web-based, user-friendly predicted nomogram was developed and validated to identify specific patients who might indeed benefit from surgical resection. The clinical availability of the established model needs to be further modified and confirmed.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 82172744), Science and Technology Commission of Shanghai Municipality (No. 21Y11913700), and Beijing Xisike Clinical Oncology Research Foundation (No. Y-2019AZQN-0511).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-997/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-997/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-997/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethical Committee of Shanghai Cancer Center, Fudan University (IRB No. 2008223-9).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y, Jheon S, Li H, et al. Results of low-dose computed tomography as a regular health examination among Chinese hospital employees. J Thorac Cardiovasc Surg 2020;160:824-831.e4. [Crossref] [PubMed]
- Laohathai S, Cho S, Yum S, et al. Clinical and functional outcomes after curative resection in octogenarians with clinical stage I non-small cell lung cancer. J Geriatr Oncol 2019;10:436-8. [Crossref] [PubMed]
- Mao Y, Gao Z, Yin Y. Complete Video-Assisted Thoracoscopic Surgery and Traditional Open Surgery for Elderly Patients With NSCLC. Front Surg 2022;9:863273. [Crossref] [PubMed]
- Committee for Scientific Affairs. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414-49. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Brunelli A, Charloux A, Bolliger CT, et al. The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg 2009;36:181-4. [Crossref] [PubMed]
- Dillman RO, Zusman DR, McClure SE. Surgical resection and long-term survival for octogenarians who undergo surgery for non-small-cell lung cancer. Clin Lung Cancer 2009;10:130-4. [Crossref] [PubMed]
- Oxnard GR, Fidias P, Muzikansky A, et al. Non-small cell lung cancer in octogenarians: treatment practices and preferences. J Thorac Oncol 2007;2:1029-35. [Crossref] [PubMed]
- Detillon DDEMA, Veen EJ. Postoperative Outcome After Pulmonary Surgery for Non-Small Cell Lung Cancer in Elderly Patients. Ann Thorac Surg 2018;105:287-93. [Crossref] [PubMed]
- Clérigo V, Hasmucrai D, Teixeira E, et al. Characterization and management of elderly and very elderly patients with non-small cell lung cancer. Clin Respir J 2020;14:683-6. [Crossref] [PubMed]
- Willén L, Berglund A, Bergström S, et al. Are older patients with non-small cell lung cancer receiving optimal care? A population-based study. Acta Oncol 2022;61:309-17. [Crossref] [PubMed]
- Saftic I, Bille A, Asemota N, et al. Risks and rewards of the surgical treatment of lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2021;33:905-12. [Crossref] [PubMed]
- Asemota N, Saftic I, Tsitsias T, et al. Quality of Life in Octogenarians After Lung Resection Compared to Younger Patients. Clin Lung Cancer 2022;23:e118-30. [Crossref] [PubMed]
- Ganti AK, Shostrom V, Alorabi M, et al. Early Stage Non-Small-Cell Lung Cancer in Octogenarian and Older Patients: A SEER Database Analysis. Clin Lung Cancer 2016;17:285-91. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Ligabue T, et al. Short- and long-term results of lung resection for cancer in octogenarians. Asian Cardiovasc Thorac Ann 2009;17:147-52. [Crossref] [PubMed]
- Vazirani J, Moraes J, Barnett S, et al. Outcomes following resection of non-small cell lung cancer in octogenarians. ANZ J Surg 2018;88:1322-7. [Crossref] [PubMed]
- Zhong D, Lin Q, Zhang J, et al. Short- and medium-term outcomes after uniportal and multiportal video-assisted thoracic surgery lobectomy in elderly patients with non-small cell lung cancer. J BUON 2021;26:1453-9. [PubMed]
- Bongiolatti S, Mazzoni F, Gonfiotti A, et al. Short and mid-term outcomes of multimodal treatment for locally-advanced non-small cell lung cancer in elderly patients. Gen Thorac Cardiovasc Surg 2020;68:1290-7. [Crossref] [PubMed]
- Mimae T, Saji H, Nakamura H, et al. Survival of Octogenarians with Early-Stage Non-small Cell Lung Cancer is Comparable Between Wedge Resection and Lobectomy/Segmentectomy: JACS1303. Ann Surg Oncol 2021;28:7219-27. [Crossref] [PubMed]
- Mimae T, Miyata Y, Tsutani Y, et al. Wedge resection as an alternative treatment for octogenarian and older patients with early-stage non-small-cell lung cancer. Jpn J Clin Oncol 2020;50:1051-7. [Crossref] [PubMed]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291:2720-6. [Crossref] [PubMed]
- Blanco R, Maestu I, de la Torre MG, et al. A review of the management of elderly patients with non-small-cell lung cancer. Ann Oncol 2015;26:451-63. [Crossref] [PubMed]
- Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol 2012;30:2036-8. [Crossref] [PubMed]
- Payne JK, Hendrix CC. Clinical trial recruitment challenges with older adults with cancer. Appl Nurs Res 2010;23:233-7. [Crossref] [PubMed]
- Saji H, Ueno T, Nakamura H, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: JACS1303. Eur J Cardiothorac Surg 2018;53:835-41. [Crossref] [PubMed]
- Nakao M, Saji H, Mun M, et al. Prognostic Impact of Mediastinal Lymph Node Dissection in Octogenarians With Lung Cancer: JACS1303. Clin Lung Cancer 2022;23:e176-84. [Crossref] [PubMed]
- Zheng C, Luo C, Xie K, et al. Distinguishing optimal esophagectomy candidates in elderly patients: A nomogram based on propensity score matching. Eur J Surg Oncol 2022;48:909-16. [Crossref] [PubMed]
- Liang H, Liu Z, Huang J, et al. Identifying optimal candidates for primary tumor resection among metastatic non-small cell lung cancer patients: a population-based predictive model. Transl Lung Cancer Res 2021;10:279-91. [Crossref] [PubMed]



