
Effect of initiative pulmonary bullectomy on the risk of post-operative pneumothorax in patients with esophageal carcinoma: a propensity score-matched analysis
Highlight box
Key findings
• Initiative pulmonary bullectomy is an effective method for the prevention of postoperative pneumothorax in esophageal cancer patients with ipsilateral pulmonary bullae.
What is known and what is new?
• IPB is an efficacious measure to prevent postoperative pneumothorax after MIE, with a statistically significant reduction in the incidence of pneumothorax.
• The simultaneous IPB does not increase other common postoperative complications of MIE.
What is the implication, and what should change now?
• Based on our experience in the current study, initiative pulmonary bullectomy during the esophagectomy is advocated in esophageal carcinoma patients complicated by ipsilateral pulmonary bullae.
Introduction
As one of the most aggressive malignancies, esophageal carcinoma accounts for 3.1% and 5.4% of global new cases and cancer deaths, ranking seventh in terms of incidence and sixth in mortality in 2020 respectively (1,2). Esophageal carcinoma which is characterized by geographic tendency presents a relatively high incidence rate in eastern Asia and eastern and southern Africa (3-6). Although the application of surgery, chemotherapy, radiotherapy, and immunotherapy has improved survival to some extent, the general outcome remains comparatively poor in terms of overall 5-year survival rates (7,8).
In the last few decades, esophagectomy based on minimally invasive techniques has shown promising results and has become the primary treatment for patients without invasion of adjacent organs or distant metastasis (9-11). However, due to the high complexity and invasive two-cavity procedure, minimally invasive esophagectomy (MIE) is still prone to induce postoperative complications, ranging from 45% to 80% of cases (12,13). Of these complications, postoperative pulmonary complications, such as pulmonary infection, atelectasis, respiratory failure, and pneumothorax, are among the major concerns. Although pneumonia is the most commonly reported postoperative respiratory complication, postoperative pneumothorax, the incidence of which remains poorly estimated, which can lead to rapid deterioration, additional invasive intervention and extended hospital stay after operation (14). In clinical practice, for some patients undergoing MIE with pulmonary bullae found during surgery, the accompanying pulmonary bullae are likely to rupture due to perioperative mechanical ventilation or active coughing, resulting in pneumothorax or postoperative continuous air leakage and even serious tension pneumothorax. Misdiagnosis or inappropriate treatment might have devastating consequences, whereas an effective and safe strategy to prevent postoperative pneumothorax could reduce the occurrence of postoperative complications and improve patient prognosis (15). Therefore, several surgeons advocated initiatively simultaneous pulmonary bullectomy during the operation. However, the effect of initiative pulmonary bullectomy (IPB) on preventing postoperative pneumothorax remains controversial and related studies have remained very limited. In this retrospective study, the effectiveness and safety of simultaneous ipsilateral IPB in esophageal carcinoma patients receiving minimally invasive esophagectomy were evaluated. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1061/rc).
Methods
Patients
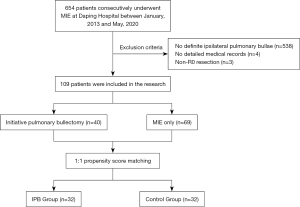
A retrospective analysis of 654 consecutive patients with esophageal carcinoma who underwent minimally invasive esophagectomy between January 2013 and May 2020 at Daping Hospital was conducted. All the patients included in this research fit the following criteria: (I) pathologically confirmed esophageal carcinoma; (II) a definite diagnosis of ipsilateral pulmonary bullae based on preoperative radiological findings or intraoperative exploration; and (III) complete medical records. Incomplete resection and only contralateral pulmonary bullae were considered exclusion criteria.
Of the 654 esophageal carcinoma patients, 109 were included in the analysis. Among the 545 patients excluded from the study, 538 patients were confirmed to have no definite ipsilateral pulmonary bullae, four patients did not have detailed medical records, and 3 had undergone incomplete esophageal tumor resection (Figure 1). All patients included in the study were restaged according to the 8th edition of the American Joint Committee on Cancer classification system. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Daping Hospital (No. 2022[159]). The need for patient consent was waived due to the retrospective nature of the study.
Surgical procedures
All operations were performed under single-lumen intubation and artificial pneumothorax. The position during surgery, incisions, esophageal mobilization and dissection as well as mediastinal lymph node dissection were performed essentially as previously described (16). After dissecting the esophagus and mediastinal lymph nodes, ipsilateral pulmonary bullectomy was performed under the same anesthesia in IPB group. The target regions selected using preoperative imaging and intraoperative findings were carefully inspected, and wedge resections of the visible bullae (Varderschueren classification III and IV stages) of the lung surface were accomplished using endostaplers. During the surgery, two chest tubes were routinely placed in the mediastinal and basal positions.
Postoperative management
As a potentially life-threatening postoperative complication, pneumothorax is the accumulation of air in the pleural space, usually due to rupture of the pulmonary bullae. During the postoperative course, pneumothorax was suspected in the presence of rapid breathing, respiratory distress, decreased blood oxygen saturation and an absence of or decrease in respiratory sounds on the affected side. A definite diagnosis was made by radiographic methods. Additional thoracic drainage was performed when pneumothorax occupied more than 30% of the hemithorax or subcutaneous emphysema was progressively exacerbated. Uniform routine postoperative management, including fluid management, pain relief, enteral and oral feeding, removal of drains and so on, was applied based on guidelines (17,18).
Statistical analysis
All statistical tests were performed using SPSS software, version 22.0 (IBM, Chicago, IL, USA). Statistical analyses were performed using Student’s t-test, the chi-square test and logistic regression analysis between the initiative pulmonary bullectomy group and the control group. To balance the heterogeneity in baseline characteristics between the two groups (IPB group vs. control group), a 1:1 PSM was performed with a caliper width of 0.05. Propensity scores were based on age, sex, smoking history, body mass index (BMI), history of chronic obstructive pulmonary disease (COPD), percent predicted forced expiratory volume in one second (FEV1%) of predicted, pleural adhesion and tumor location. P<0.05 was considered statistically significant.
Results
Patient characteristics
Before PSM, totals of 40 (36.70%) and 69 (63.30%) esophageal carcinoma patients were assigned to the IPB group and control group, respectively, according to whether initiative pulmonary bullectomy was performed. The IPB group consisted of 40 men, with a mean age of 62.58±6.98 (range, 48–77) years old, whereas the control group included more female patients (n=6, 8.70%, P=0.084) with the mean age was 64.94±8.17 (range, 46–76, P=0.380) years old. Patients receiving IPB had relatively poorer pulmonary function [both percent predicted forced vital capacity (FVC%) and FEV1%, 95.22%±14.99% vs.100.84%±19.89% and 86.25%±17.58% vs. 93.34%±24.95%, P=0.017 and 0.032]. There were 31 patients (77.50%) in IPB group and 53 patients (76.81%) in control group detected bullae by preoperative CT (P=1.000). The distribution of the pathologic T stage between the two groups differed significantly (P=0.028). In addition, more tumors located in the middle thoracic esophagus were found in the control group (35.00% vs. 59.42%, P=0.049). The proportion of patients who had smoking history in the IPB group was slightly greater than that in the CG, but the difference was not statistically significant (97.50% vs. 85.51%, P=0.053). Furthermore, no obvious differences were observed in body mass index, drinking history, underlying diseases, neoadjuvant therapy, pathological type, pathological N staging, tumor differentiation or pleural adhesion between the two groups (Table 1).
Table 1
| Characteristics | Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|---|
| IPB (n=40) | CG (n=69) | P value | IPB (n=32) | CG (n=32) | P value | ||
| Age (years) | 62.58±6.98 | 64.94±8.17 | 0.380 | 63.16±7.51 | 63.38±8.16 | 0.610 | |
| Gender | 0.084 | 1.000 | |||||
| Male | 40 (100.0) | 63 (91.30) | 32 (100.0) | 32 (100.00) | |||
| Female | 0 | 6 (8.70) | 0 | 0 | |||
| BMI (kg/m2) | 21.74±2.93 | 21.66±2.61 | 0.344 | 21.24±2.68 | 21.79±2.49 | 0.669 | |
| Smoking history | 39 (97.50) | 59 (85.51) | 0.053 | 31 (96.88) | 31 (96.88) | 1.000 | |
| Drinking history | 28 (70.00) | 41 (59.42) | 0.307 | 21 (65.63) | 21 (65.63) | 1.000 | |
| Hypertension | 9 (22.50) | 16 (23.19) | 1.000 | 6 (18.75) | 4 (12.50) | 0.732 | |
| Diabetes | 3 (7.50) | 5 (7.25) | 1.000 | 3 (9.38) | 4 (12.50) | 1.000 | |
| COPD | 6 (15.00) | 22 (31.88) | 0.069 | 6 (18.75) | 9 (28.13) | 0.556 | |
| Arrhythmia | 1 (2.50) | 4 (5.80) | 0.650 | 1 (3.13) | 2 (6.25) | 1.000 | |
| PFT | |||||||
| FVC% (%) | 95.22±14.99 | 100.84±19.89 | 0.017 | 95.58±14.02 | 95.44±18.37 | 0.090 | |
| FEV1% (%) | 86.25±17.58 | 93.34±24.95 | 0.032 | 86.31±17.46 | 94.36±23.31 | 0.315 | |
| MVV% (%) | 90.95±21.28 | 88.62±25.80 | 0.368 | 91.44±22.91 | 91.61±25.75 | 0.781 | |
| First discovered by | 1.000 | 0.732 | |||||
| Preoperative CT | 31 (77.50) | 53 (76.81) | 28 (87.50) | 26 (81.25) | |||
| Intraoperative exploration | 9 (22.50) | 16 (23.19) | 4 (12.50) | 6 (18.75) | |||
| Neoadjuvant | 10 (25.00) | 10 (14.49) | 0.172 | 10 (31.25) | 8 (25.00) | 0.782 | |
| Time of operation (min) | 323.38±52.24 | 321.98±57.37 | 0.904 | 324.69±51.38 | 327.19±61.32 | 0.862 | |
| Tumor location | 0.049 | 0.130 | |||||
| Upper | 12 (30.00) | 13 (18.84) | 9 (28.13) | 6 (18.75) | |||
| Middle | 14 (35.00) | 41 (59.42) | 10 (31.25) | 18 (56.25) | |||
| Lower | 14 (35.00) | 15 (21.74) | 13 (40.62) | 8 (25.00) | |||
| Pathological types | 0.181 | 1.000 | |||||
| Squamous cell | 40 (100.00) | 66 (95.65) | 32 (100.00) | 31 (96.88) | |||
| Adenocarcinoma | 0 | 3 (4.45) | 0 | 1 (3.12) | |||
| Pathological T classification | 0.028 | 0.100 | |||||
| Tis | 3 (7.50) | 0 (0) | 3 (9.37) | 0 (0) | |||
| T1 | 6 (15.00) | 12 (17.39) | 4 (12.50) | 3 (9.37) | |||
| T2 | 15 (37.50) | 14 (20.29) | 12 (37.50) | 7 (21.88) | |||
| T3 | 13 (32.50) | 38 (55.07) | 12 (37.50) | 20 (62.50) | |||
| T4 | 3 (7.50) | 5 (7.25) | 1 (3.13) | 2 (6.25) | |||
| Pathological N classification | 0.477 | 0.657 | |||||
| N0 | 22 (55.00) | 33 (47.83) | 17 (53.12) | 13 (40.63) | |||
| N1 | 7 (17.50) | 17 (24.64) | 5 (15.63) | 7 (21.87) | |||
| N2 | 7 (17.50) | 16 (23.19) | 6 (18.75) | 9 (28.12) | |||
| N3 | 4 (10.00) | 3 (4.35) | 4 (12.50) | 3 (9.38) | |||
| Tumor differentiation | 0.726 | 1.000 | |||||
| G1 | 13 (32.50) | 16 (23.19) | 10 (31.25) | 10 (31.25) | |||
| G2 | 19 (47.50) | 38 (55.07) | 15 (46.88) | 15 (46.88) | |||
| G3 | 5 (12.50) | 8 (11.59) | 4 (12.50) | 4 (12.50) | |||
| NA | 3 (7.50) | 7 (10.15) | 3 (9.37) | 3 (9.37) | |||
| Pleural adhesion | 13 (32.50) | 23 (33.33) | 0.929 | 12 (37.50) | 16 (50.00) | 0.450 | |
Data are represented as mean ± SD or n (%). IPB, initiative pulmonary bullectomy; CG, control group; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PFT, pulmonary function test; FVC, forced vital capacity; FEV1, first second forced expiratory volume; MVV, maximum ventilatory volume; CT, computed tomography.
After PSM, the cohorts were narrowed to 32 patients in each group. The baseline data, such as smoking history, FEV1% of predicted, pathologic T stage and tumor location, were compared between the two groups (Table 1). All further statistical analyses were performed on this population.
Postoperative complications
As shown in Table 2, the total complication rates in the two groups were 43.75% and 56.25% respectively (P=0.454), and the most frequent complication in both groups was postoperative pneumonia (37.50% vs. 43.75%, P=0.799). No significant difference was found between the two groups in terms of the incidence of anastomotic leakage (6.25% vs. 3.13%, P=1.000), arrhythmia (3.13% vs. 3.13%, P=1.000), or chylothorax (0% vs. 3.13%, P=1.000). Postoperative pneumothorax occurred in only one patient in the IPB group (3.13%) and 13 patients in the control group (40.63%), including 11 patients (34.38%) with surgery-ipsilateral pneumothorax, with a significant difference (P<0.001). The mean time of occurrence of postoperative pneumothorax was 3.44±1.68 days (range, 1–11 days). Except for one patient who underwent thoracentesis in the CG, the other 13 patients in both groups were treated with additional closed thoracic drainage, and no reoperations or hospital mortalities were reported among these patients. In addition, the length of postoperative hospital stay in the control group (16.13±8.35 days) was much longer than that of the remaining patients who received initiative pulmonary bullectomy (13.28±6.32 days, P=0.045).
Table 2
| Characteristics | Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|---|
| IPB (n=40) | CG (n=69) | P value | IPB (n=32) | CG (n=32) | P value | ||
| Pneumonia | 17 (42.50) | 31 (44.93) | 0.844 | 12 (37.50) | 14 (43.75) | 0.799 | |
| Anastomotic leak | 3 (7.50) | 4 (5.80) | 0.706 | 2 (6.25) | 1 (3.13) | 1.000 | |
| Postoperative arrhythmia | 2 (5.00) | 2 (2.90) | 0.623 | 1 (3.13) | 1 (3.13) | 1.000 | |
| Chylothorax | 0 | 1 (1.45) | 1.000 | 0 | 1 (3.13) | 1.000 | |
| Pneumothorax | <0.001 | <0.001 | |||||
| Ipsilateral | 1 (2.50) | 27 (39.13) | 1 (3.13) | 11 (34.38) | |||
| Contralateral | 0 | 6 (8.70) | 0 | 2 (6.25) | |||
| Length of postoperative stay, days | 13.63±6.10 | 17.80±11.65 | <0.001 | 13.28±6.32 | 16.13±8.35 | 0.045 | |
Data are represented as mean ± SD or n (%). IPB, initiative pulmonary bullectomy; CG, control group.
Univariate and multivariate logistic regression analysis
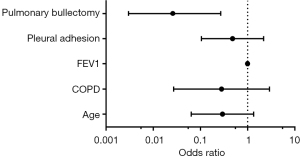
Finally, univariate and multivariate logistic analyses were performed to identify the independent factors associated with postoperative pneumothorax after MIE (Table 3). Initial univariate regression analyses showed that pulmonary bullectomy was significantly linked to a reduced risk of developing postoperative pneumothorax. In the multivariate analysis, the removal of ipsilateral bullae was also associated with a lower risk (OR 0.030; 95% CI: 0.003–0.338; P=0.005) of incident postoperative pneumothorax, adjusting for age, COPD history, FEV1 and pleural adhesion. Therefore, the initiation of pulmonary bullectomy in patients with esophageal carcinoma complicated by ipsilateral pulmonary bullae was an independent protective factor for postoperative pneumothorax (Figure 2).
Table 3
| Characteristics | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P Value | ||
| Age (<60 vs. ≥60 years) | 0.316 | (0.092–1.083) | 0.067 | 0.293 | (0.064–1.343) | 0.114 | |
| Gender | 0.280 | (0–3.509) | 1.000 | ||||
| BMI, kg/m2 | 0.651 | ||||||
| 18.5–23.99 | 1 | – | – | ||||
| <18.5 | 3.800 | (0.475–30.419) | 0.208 | ||||
| 24–27.99 | 0.950 | (0.174–5.194) | 0.953 | ||||
| ≥28 | 0 | – | 0.999 | ||||
| Smoking history | 1.099 | (0–1.549) | 0.999 | ||||
| COPD | 0.474 | (0.093–2.409) | 0.368 | 0.280 | (0.027–2.903) | 0.286 | |
| FVC | 1.003 | (0.967–1.041) | 0.866 | ||||
| FEV1 | 1.023 | (0.991–1.057) | 0.161 | 0.997 | (0.955–1.040) | 0.882 | |
| MVV | 1.004 | (0.980–1.029) | 0.741 | ||||
| Neoadjuvant | 1.029 | (0.277–3.826) | 0.966 | ||||
| Tumor location | 0.231 | ||||||
| Upper | 1 | – | – | ||||
| Middle | 3.079 | (0.570–16,633) | 0.191 | ||||
| Lower | 1.083 | (0.158–7.435) | 0.935 | ||||
| T classification | 0.212 | ||||||
| Tis | 1 | – | – | ||||
| T1-2 | 1.000 | – | 0.999 | ||||
| T3-4 | 1.000 | – | 0.999 | ||||
| N classification | 0.585 | ||||||
| N0 | 1 | – | – | ||||
| N1 | 1.000 | (0.166–6.028) | 1.000 | ||||
| N2 | 2.500 | (0.592–10.555) | 0.212 | ||||
| N3 | 2.000 | (0.299–13.375) | 0.475 | ||||
| Surgery approach | 1.009 | (0.000–1.347) | 1.000 | ||||
| Pleural adhesion | 0.939 | (0.288–3.159) | 0.939 | 0.480 | (0.105–2.187) | 0.343 | |
| Bullectomy | 0.062 | (0.007–0.513) | 0.010 | 0.030 | (0.003–0.338) | 0.005 | |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, first second forced expiratory volume; MVV, maximum ventilatory volume; CI, confidence interval.
Discussion
Over the past few years, surgical and anesthetic techniques have shown visible improvement; however, the complication rate seems to continue to stagnate due to the high complexity of the esophagectomy procedure. As reported in previous studies, pulmonary complications after esophagectomy or MIE, mainly including pneumonia, atelectasis, respiratory distress, and pneumothorax, are the major postoperative complications reported in up to 40% of esophageal carcinoma patients (19,20). Among them, pneumothorax is a relatively frequent complication after MIE. This is probably a consequence of parts of esophageal carcinoma patients complicated by pulmonary bullae or emphysema, intraoperative manipulation especially adhesiolysis, improper mechanical ventilation and over-vigorous cough after the MIE. Several previous studies have reported pneumothorax after minimally invasive esophagectomy with an incidence of 0.79% to 3.4% (14,21,22). However, the present study showed an 31.19% incidence of postoperative pneumothorax after surgery in patients with a definite diagnosis of pulmonary bullae or emphysema, compared to 5.19% in the whole cohort. The possible explanations for the higher incidence of postoperative pneumothorax compared with previous studies are as follows: (I) the higher tobacco use rates in the present cohort (76.76%); (II) more patients having a history of chronic respiratory disease (44.19%); and (III) overemphasis on active cough during preoperative publicizing and education.
Due to surgical trauma, pain, postoperative dysfunction of respiratory muscles and diminished airway protection, lung function in these postoperative patients is likely to have been compromised; therefore, postoperative pneumothorax often rapidly presents as a potentially life-threatening disease, requiring immediate intervention (15,23). These invasive manipulations, either manual aspiration with a needle or additional chest tubes connected to a water seal drainage device, will undoubtedly increase the unpleasant experience of patients, extend the length of hospital stay and impede postoperative recovery to some extent. For this reason, further exploration of promising prophylactic methods for postoperative pneumothorax in esophageal carcinoma patients is essential and quite urgent to reduce morbidity and mortality and improve short-term prognosis.
In this study, we compared the postoperative pneumothorax rates and other short-term complications between the IPB group and control group. Our data showed that initiative pulmonary bullectomy during surgery is an efficacious measure to prevent postoperative pneumothorax after minimally invasive esophagectomy, with a statistically significant reduction in the incidence of pneumothorax. This result is similar to the study by Zhang et al. which indicated that prophylactic bullectomy should be performed as a routine procedure when a definite diagnosis is made based on intraoperative exploration without considering whether the bubble is ruptured (24). Moreover, Hu et al. summarized a clinical experience in the treatment of postoperative pneumothorax in 155 patients who received esophagectomy and suggested that esophageal tumors and pulmonary bullae should be managed at same time if possible (25). In addition, since no significant difference was detected between the two groups in other short-term complications, we believe that the prolonged hospital stays between the two groups reflects the impediment of postoperative pneumothorax during recovery in patients with esophageal carcinoma. Although some previous reports have shown the usefulness of prophylactic bullectomy for esophageal carcinoma patients, the current study is the first research to evaluate the effect of initiative intraoperative pulmonary bullectomy on preventing postoperative pneumothorax based on a propensity score-matched analysis and represents the largest study to date.
The other question that should receive much attention is whether the safety of minimally invasive esophagectomy is decreased by routine-initiative pulmonary bullectomy. Tan et al. previously reported their experience in treating esophageal and cardiac cancer patients with coexisting severe emphysema by combining esophageal tumor resection with simultaneous unilateral lung volume reduction surgery in selected patients, with favorable outcomes (26). Similarly, Tang et al. also elucidated the simultaneous lung volume reduction surgery could not only increase the chance of receiving surgical therapy, but also improve the postoperative quality of life of esophageal carcinoma patients complicated by emphysema (27). In fact, resection of heterogenous lung parenchyma without respiratory function could counteract the effect of hyperinflation, providing decreased work to breathe and improved alveolar gas exchange (28). Coupled with the current results, we believe that simultaneous pulmonary bullectomy does not induce unacceptable relative morbidity and mortality. In contrast, it could have some postoperative benefits and survival advantages for selected patients. Although no extra air leakage was observed after surgical resection of diseased lung in this research, we still suggest that the surgical margin should be kept no less than 10 mm from the base of the lesion to avoid possible air leakage due to the absence of water tests or pleurodesis procedures. In our study, the incidences of postoperative pneumonia, anastomotic leakage, arrhythmia, and chylothorax in both groups were similar, demonstrating that simultaneous-initiative pulmonary bullectomy could be performed in combination with esophageal resection in esophageal carcinoma patients with coexisting pulmonary bullae, with acceptable morbidity and without mortality. However, the differences between the IPB and control groups in long-term complications, postoperative recovery of lung function and overall survival require further study.
In the present study, the retrospective nature, limited number of patients and single institution constituted major limitations. Although PSM was performed to improve comparability between the two groups, the results could be affected by the smaller sample sizes and potential selection biases. Therefore, a larger multicenter and randomized study with follow-up is needed to further validate our results.
Conclusions
The present study revealed that initiative pulmonary bullectomy is an effective and safe method for reducing the occurrence of postoperative pneumothorax and it does not increase other common postoperative complications. We recommend performing simultaneous and initiative pulmonary bullectomy during MIE for patients with ipsilateral pulmonary bullae who suffer from esophageal carcinoma. The present study might provide a novel prophylactic strategy for postoperative pneumothorax, improve the postoperative outcomes of esophageal surgery, and contribute to enhanced recovery after surgery.
Acknowledgments
The authors are indebted to all of the people whose names were not included in the author list but who participated in our study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1061/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1061/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1061/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1061/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Daping Hospital (No. 2022[159]). The need for patient consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- He F, Wang J, Liu L, et al. Esophageal cancer: trends in incidence and mortality in China from 2005 to 2015. Cancer Med 2021;10:1839-47. [Crossref] [PubMed]
- Short MW, Burgers KG, Fry VT. Esophageal Cancer. Am Fam Physician 2017;95:22-8. [PubMed]
- Zheng RS, Zhang SW, Zeng HM, et al. Cancer incidence and mortality in China, 2016. J Nat Cancer Cent 2022;2:1-9. [Crossref]
- Uhlenhopp DJ, Then EO, Sunkara T, et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol 2020;13:1010-21. [Crossref] [PubMed]
- He H, Chen N, Hou Y, et al. Trends in the incidence and survival of patients with esophageal cancer: A SEER database analysis. Thorac Cancer 2020;11:1121-8. [Crossref] [PubMed]
- Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol 2016;31:1141-6. [Crossref] [PubMed]
- Sakamoto T, Fujiogi M, Matsui H, et al. Comparing Perioperative Mortality and Morbidity of Minimally Invasive Esophagectomy Versus Open Esophagectomy for Esophageal Cancer: A Nationwide Retrospective Analysis. Ann Surg 2021;274:324-30. [Crossref] [PubMed]
- Borggreve AS, Kingma BF, Domrachev SA, et al. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci 2018;1434:192-209. [Crossref] [PubMed]
- Gottlieb-Vedi E, Kauppila JH, Malietzis G, et al. Long-term Survival in Esophageal Cancer After Minimally Invasive Compared to Open Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg 2019;270:1005-17. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- DeMaio A, Semaan R. Management of Pneumothorax. Clin Chest Med 2021;42:729-38. [Crossref] [PubMed]
- Guo W, Zhao YP, Jiang YG, et al. Prevention of postoperative chylothorax with thoracic duct ligation during video-assisted thoracoscopic esophagectomy for cancer. Surg Endosc 2012;26:1332-6. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Li Y. Strategy and prospective of enhanced recovery after surgery for esophageal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2016;19:965-70. [PubMed]
- Klevebro F, Elliott JA, Slaman A, et al. Cardiorespiratory Comorbidity and Postoperative Complications following Esophagectomy: a European Multicenter Cohort Study. Ann Surg Oncol 2019;26:2864-73. [Crossref] [PubMed]
- Chevallay M, Jung M, Chon SH, et al. Esophageal cancer surgery: review of complications and their management. Ann N Y Acad Sci 2020;1482:146-62. [Crossref] [PubMed]
- Gillinov AM, Heitmiller RF. Strategies to reduce pulmonary complications after transhiatal esophagectomy. Dis Esophagus 2017;11:43-7. [Crossref] [PubMed]
- Uchihara T, Yoshida N, Baba Y, et al. Risk factors for pulmonary morbidities after minimally invasive esophagectomy for esophageal cancer. Surg Endosc 2018;32:2852-8. [Crossref] [PubMed]
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010;19:217-9. [Crossref] [PubMed]
- Zhang BL, Yi Y, Yang SF. Prevention and Treatment of the Spontaneous Pneumothorax after the Esophagus Carcinoma Operation. Academic Journal of Kunming Medical College 2003;24:92-3.
- Hu CM, Zhou FY, Geng MF, et al. Cause analysis and prevention and treatment of 155 cases of postoperative pneumothorax for esophageal cancer. Chinese Journal of Practical Diagnosis and Treatment 2007;21:798-9.
- Tan QY, Wang RW, Jiang YG, et al. Lung volume reduction surgery allows esophageal tumor resection in selected esophageal carcinoma with severe emphysema. Ann Thorac Surg 2006;82:1849-56. [Crossref] [PubMed]
- Tang YJ, Wang CY, Wang CD, et al. Clinical study of simultaneous lung volume reduction surgery during resection of pulmonary or esophageal neoplasms. Chin Med J (Engl) 2009;122:2973-6. [PubMed]
- Fessler HE, Scharf SM, Ingenito EP, et al. Physiologic basis for improved pulmonary function after lung volume reduction. Proc Am Thorac Soc 2008;5:416-20. [Crossref] [PubMed]


