
Esophageal stenting with minimally-invasive surgical intervention for delayed spontaneous esophageal perforation
Highlight box
Key findings
• Esophageal stenting with minimally-invasive surgical intervention was feasible and effective for delayed spontaneous esophageal perforation, a challenging clinical problem which has traditionally carried a high rate of morbidity and mortality.
What is known and what is new?
• Delayed spontaneous esophageal perforation can lead to high mortality. Primary repair is the standard surgical approach but it is not always feasible. Currently, there is no consensus for the management of delayed spontaneous esophageal perforations.
• Endoscopic esophageal stent placement with extraluminal sutures to prevent stent migration, thoracic decortication with chest tube drainage, gastric decompression, and jejunostomy tube placement for early nutrition was feasible and effective in the treatment of delayed spontaneous esophageal perforation.
What is the implication, and what should change now?
• This technique offers a less invasive treatment approach for a challenging clinical problem which has traditionally carried a high rate of morbidity and mortality.
Introduction
Spontaneous esophageal perforation, also known as Boerhaave’s Syndrome, most commonly occurs as a full-thickness tear along the left aspect of the distal intrathoracic esophagus near the gastroesophageal junction (GEJ). Esophageal perforation is a surgical emergency with a high morbidity and mortality. Timely primary repair within 24-hour after symptom onset carries good outcomes (1,2). However, delayed diagnosis and treatment of esophageal perforation is not uncommon and can lead to high mortality due to ongoing mediastinal contamination (2-4). Direct repair to prevent continued leakage from the esophagus is not always feasible due to severe tissue necrosis.
Esophageal stenting can provide therapeutic benefits in the management of benign and malignant esophageal diseases (5,6). Several studies have shown the safety and efficacy of stent placement in the treatment of esophageal strictures, including self-expanding plastic (SEPS) and metal stents (SEMS) (4-15). Fully covered self-expanding metal stents (FCSEMS) are preferable over partially covered self-expanding metal stents (PCSEMS) in benign esophageal perforation due to less complications from tissue ingrowth and better coverage of the perforated area (5,12). However, benign lesions, distal esophageal perforation, and FCSEMS are risk factors for stent migration which may result in severe complications (13,16-18).
In this study, we review our experience utilizing a hybrid approach combining endoscopic esophageal stent placement with extraluminal sutures to prevent stent migration and minimally invasive surgical drainage to treat delayed spontaneous esophageal perforations. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1316/rc).
Methods
Patients
This retrospective, observational study enrolled all patients referred to the thoracic surgery service for delayed spontaneous esophageal perforation at Chang Gung Memorial Hospital (Linkou, Taoyuan) between September 2018 and March 2021. Delayed spontaneous esophageal perforation was defined as an interval between initial symptoms and a confirmed perforation of more than 24 hours. All patients with recent upper endoscopy, esophageal instrumentation, recent intrathoracic or upper abdominal surgery, or perforation associated with esophageal malignancy, achalasia, or paraesophageal hernia were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Chang Gung Memorial Hospital (No. 202100306B0) and informed consent was waived for this retrospective study.
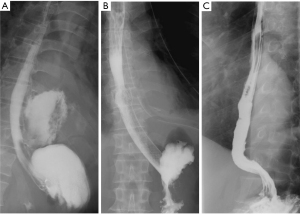
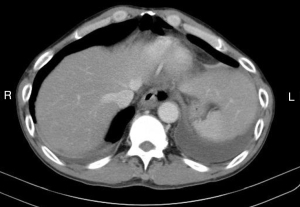
The diagnosis of an esophageal perforation was documented on esophagram with water soluble contrast which showed the extravasation of oral contrast identifying the location of the perforation (Figure 1). The Pittsburgh perforation severity score (PSS) was used to classify the esophageal perforation (19). The PSS was determined according to the following scale: 1= age >75 years, tachycardia, leukocytosis, or pleural effusion; 2= fever >38.5 ℃, non-contained leak, respiratory compromise, or time to diagnosis >24 hours; and 3= presence of cancer or hypotension. Low PSS, intermediate PSS and high PSS groups were defined as PSS <2, PSS 3–5, and PSS >5, respectively. All patients underwent computed tomography imaging from the neck to the abdomen to evaluate for contamination of the mediastinum, pleural cavity, or peritoneal cavity (Figure 2).
Surgery
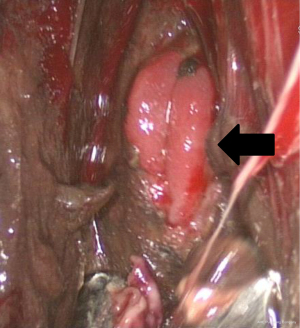
All procedures were done in the operating room under general anesthesia. The contaminated area was adequately drained by video-assisted thoracic surgery (VATS) in order to achieve optimal infection control. The patient was first placed in a lateral position, and single lung ventilation was accomplished using a double-lumen endotracheal tube. VATS decortication was performed through two ports. The working port was placed in the 5th intercostal space in the mid axillary line. The camera port was inserted in the 7th or 8th intercostal space. Once the pleural cavity was entered, all pleural effusion, pus, debris and fibrinous tissue were evacuated. Pneumonolysis was done to separate all adherent lung from the pleural surface. The esophageal perforation site was then evaluated under VATS, including the size and location of the perforation as well as the viability of the surrounding tissues (Figure 3). The visceral pleural peel was removed using ring forceps or a curette to prevent entrapment of the lung. At the end of the procedure, we copiously irrigated the pleural cavity with saline. Chest tubes were placed adjacent to the perforation and at a dependent site to achieve adequate drainage and prevent the accumulation of infected material.
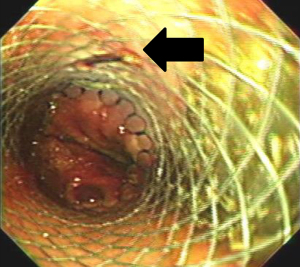
The patient was positioned supine for the second portion of the procedure. Flexible esophagoscopy was performed to characterize the location and extent of the esophageal perforation. We avoided esophageal stent placement in the presence of obvious circumferential esophageal necrosis or a long perforation unable to be covered by a single stent. The size of the esophageal stent was chosen according to the diameter of the esophagus on the preoperative esophagram. All esophageal stents (WallFlex; Boston Scientific, Natick, MA, USA) were placed under fluoroscopic guidance. During mini-laparotomy for feeding tube placement, stents were sutured to the esophagus extraluminally using absorbable polydioxanone (PDS; Ethicon US, LLC, Guaynabo, Puerto Rico) or polyglycolic acid (Dexon; Medtronic, Minneapolis, MN, USA) sutures. The sutures were placed at the level of the abdominal esophagus and passed through all layers of the esophagus and the stent to prevent stent migration. This extraluminal suture was confirmed on endoscopic surveillance (Figure 4). For adequate coverage of the perforation, the distal end of the stent must be situated 2 cm below the GEJ. A feeding jejunostomy was then placed for early enteral nutrition. The stomach was decompressed with either a gastrostomy or nasogastric tube.
Post-stent placement, an esophagram was obtained to confirm adequate coverage of the esophageal perforation once the patient was able to tolerate the examination (Figure 1). Patients were started on a liquid diet and then gradually advanced to a soft diet in the absence of any esophageal leakage. All esophageal stents were removed by 8–12 weeks after initial stent insertion. The timing of stent removal was individualized according to the extent of the perforation and the patient’s nutritional condition as well as control of any ongoing infection. All esophageal stents were removed by endoscopy using sedation. An esophagram was performed within 24 hours after esophageal stent removal to evaluate for any persistent esophageal leak (Figure 1). All patients were assessed for dysphagia and adequate oral nutrition for at least 6 months.
Statistical analysis
A descriptive analysis was used for the variables in this study. Counts and percentages were used for categorical variables while median and interquartile range were shown for continuous variables.
Results
During the 54-month study period, 5 patients were identified with delayed spontaneous esophageal perforation. All perforations were located in the distal esophagus. Most patients (4/5, 80%) were male, and their mean age was 58 years (range, 48 to 86 years). The mean duration between symptoms and diagnosis was 5 days (range, 1 to 14 days). The mean interval between symptoms and esophageal stent insertion was 7 days (range, 2 to 18 days). All patients were classified in the high PSS group with a mean PSS of 11 (range, 8 to 14). No patients had a known malignancy, achalasia, or paraesophageal hernia (Table 1).
Table 1
| Variables | Patients | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age, years | 86 | 58 | 48 | 49 | 48 |
| Gender | M | M | M | F | M |
| Charlson comorbidity index | 4 | 2 | 0 | 3 | 0 |
| Time to diagnosis, days | 6 | 4 | 2 | 14 | 1 |
| Time to esophageal stent, days | 7 | 6 | 3 | 18 | 2 |
| Location of the perforation | Distal | Distal | Distal | Distal | Distal |
| Spontaneous perforation | Yes | Yes | Yes | Yes | Yes |
| Shock | Yes | No | No | Yes | Yes |
| Presence of cancer | No | No | No | No | No |
| PSS score | 12 | 10 | 8 | 14 | 13 |
PSS, Pittsburgh Perforation Severity.
All patients received a FCSEMS with suture fixation, feeding jejunostomy, and thoracoscopic decortication. One patient received a gastrostomy tube for decompression whereas the others had a nasogastric tube (Table 2).
Table 2
| Procedures | N [%] |
|---|---|
| Esophageal stenting | |
| FCSEMS | 5 [100] |
| PCSEMS | 0 [0] |
| Esophageal stent fixation sutures | |
| Polyglycolic acid (Dexon) | 3 [60] |
| PDS | 2 [40] |
| Decompression | |
| Gastrostomy | 1 [20] |
| Nasogastric tube | 4 [80] |
| Feeding jejunostomy | 5 [100] |
| Drainage method | |
| Chest tube only | 0 [0] |
| Thoracoscopic decortication | 5 [100] |
All data were presented as number (%). FCSEMS, fully covered self-expandable metal stents; PCSEMS, partially covered self-expandable metal stents; PDS, polydioxanone.
Adequate coverage of the esophageal perforation was confirmed in all patients on esophagram. The median length of stay in the intensive care unit and mechanical ventilation was 4 days and 1 day. There was no esophageal stent migration. The median length of hospital stay was 33 days. The median time to oral nutrition and esophageal stent removal was 43 and 66 days. Three patients (60%) had postoperative complications including respiratory failure [1], sepsis [1], arrhythmia [1], gastrointestinal bleeding [1], and prolonged ileus [1]. Two patients had ongoing infection and needed a prolonged course of antibiotic treatment. Among these patients, one needed another surgical drainage procedure due to uncontrolled infection and tracheostomy placement for respiratory failure. All patients experienced mild chest discomfort and gastroesophageal reflux which were well-controlled with proton-pump inhibitors and strict aspiration precautions. These symptoms resolved after esophageal stent removal. No esophageal stents needed to be replaced, and there was no hospital mortality.
All patients were discharged without chest tubes or antibiotics. All patients were able to tolerate a regular diet once the stent was removed. One fragile patient continued to require a jejunostomy tube for supplemental nutrition due to swallowing dysfunction while the jejunostomy tube was removed during follow-up for the other four patients. Two patients had symptoms of dysphagia which resolved without any additional interventions. No patients developed an esophageal stricture or required esophageal dilations. There were no 30-day or 6-month mortality (Table 3).
Table 3
| Outcomes | N (%) or median (IQR) |
|---|---|
| Adequate coverage of perforation | 5 (100.0) |
| Time to oral nutrition, days | 43 (17.5, 81.0) |
| Time to stent removal, days | 66 (55.5, 77.5) |
| Length of stay, days | 33 (24.0, 77.5) |
| Length of ICU stay, days | 4 (1.0, 26.5) |
| Length of mechanical ventilation, days | 1 (1.0, 19.0) |
| Stent migration | 0 (0) |
| Morbidity | 3 (60.0) |
| Mortality | 0 (0) |
Categorical data were presented as n (%); continuous data were expressed as median (IQR). IQR, interquartile range; ICU, intensive care unit.
Discussion
Delayed spontaneous esophageal perforation is a challenging surgical emergency. In this study, we proposed a hybrid approach combining endoscopic esophageal stent placement with extraluminal sutures to prevent stent migration, thoracoscopic decortication with chest tube drainage, gastric decompression, and jejunostomy tube placement for early nutrition. There was no mortality in this cohort, and all patients were able to resume oral nutrition with esophageal preservation after stent removal.
Several studies have shown the safety and efficiency of esophageal stent placement in the treatment of benign esophageal perforation (4-10). The most common site of spontaneous esophageal perforation was the left aspect of the distal esophagus near the GEJ. Dickinson et al. suggested that the esophageal stent should be placed 2–4 cm proximal and distal to the perforation for adequate coverage (20). For distal esophageal perforations, the stent often has to extend across the GEJ to get adequate coverage. Due to the size of the gastric lumen and necrotic tissue at the distal aspect of the perforation, finding a good distal landing zone for the stent can be challenging. As a result, the risk of stent migration is increased, and it is important to secure the stent to the esophagus.
There are various esophageal stents available for coverage of benign esophageal perforations including SEPS, FCSEMS, and PCSEMS (4-15). However, the risk of stent migration is higher with a SEPS than a FCSEMS or PCSEMS (5,13,14). Prolonged stent placement for benign esophageal disease is not recommended due to increased stent-associated complications including stent erosion with bleeding or fistula formation (21). Furthermore, tissue ingrowth occurs more frequently with PCSEMS compared to FCSEMS (5,12,13,15). The resulting granulation tissue increases the difficulty of stent retrieval and the risk of esophageal perforation (15,22,23). As a result, we recommend the placement of FCSEMS for managing benign esophageal perforations.
The radial force of the esophageal stent, particularly the flared portions at the proximal and distal ends, help to keep the stent in place. However, stent migration may be more common with a benign esophageal perforation due to the lack of a stricture to anchor the stent in place. Non-malignant etiology, distal esophageal perforation, and a FCSEMS were risk factors for stents migration (13,15,16). Previous studies have reported a rate of stent migration between 16% to 29% with benign esophageal perforations (9,10,13-15). Migrated stents may cause life-threating complications and need further endoscopic or surgical interventions to retrieve the stent (13,14,16-18). In our cohort, we chose to place FCSEMS across the GEJ in an attempt to cover the distal esophageal perforation. Due to the increased risk for stent migration, the distal end of the stent was secured to the esophageal wall extraluminally with absorbable suture. There was no stent migration, and all stents were successfully removed endoscopically.
The need for gastric decompression via nasogastric tube or gastrostomy tube with an esophageal perforation is unclear. Cameron et al. claimed that a nasogastric tube was not necessary in esophageal perforation since a nasogastric tube may increase the risk of gastroesophageal reflux and therefore delay esophageal healing (24). Other studies recommend gastric decompression to reduce persistent contamination (1,3). Early enteral nutrition and aggressive debridement and drainage of any infected or nonviable tissue and fluid are critical in the management of esophageal perforations (11,25). In the current study, all patients underwent gastric decompression with either a nasogastric or gastrostomy tube placement due to the increased risk of delayed gastric emptying in critically ill patients. Furthermore, a feeding jejunostomy was placed for early enteral nutrition, and thoracoscopic decortication was performed to drain all infected material.
Based on the results of a large international study, the Pittsburgh Severity Score (PSS) was proposed to guide treatment of esophageal perforations based on the severity of the perforation (19). The high-risk groups (PSS >5) had increased morbidity and mortality rates (82.5% and 37.5% respectively). Furthermore, 14.2% of patients underwent esophagectomy as part of their management. The predictive value of the PSS is better in patients with a spontaneous esophageal perforation (26). Despite all patients in the current study having a high PSS (range, 8 to 14), there were no in-hospital deaths. In addition, all patients resumed oral nutrition with esophageal preservation.
Endoscopic vacuum therapy (EVT) is another emerging treatment modality for esophageal perforation (27,28). However, reports of EVT in delayed spontaneous esophageal perforation are limited. In general, multiple additional procedures (mean 6.4–7.4) were required for EVT management of esophageal perforation (27,28). In addition, 30% of patients had dysphagia after recovery following EVT (28). In our hybrid approach, aside from one patient who needed another surgical drainage procedure due to uncontrolled infection and tracheostomy placement for respiratory failure, no additional interventions were required to manage the esophageal perforation. All patients resumed oral intake without dysphagia except one who had swallowing dysfunction.
The treatment approach demonstrated in the current study appears to be safe and effective. However, there were some limitations with this study. The patient cohort was small for this relatively rare condition. Furthermore, selection bias may exist in this retrospective study. Larger, prospective studies are needed to validate our findings.
Conclusions
Endoscopic esophageal stent placement with extraluminally sutures to prevent stent migration, thoracoscopic decortication with chest tube drainage, gastric decompression, and jejunostomy tube placement for early nutrition was feasible and effective in the treatment of delayed spontaneous esophageal perforation. FCSEMS placement across the gastroesophageal junction provided adequate coverage of the esophageal perforation and prevented continued mediastinal contamination. There were no cases of stent migration after securing the distal end of the stent to the esophagus with absorbable suture. All stents were successfully removed without complications and with complete healing of the esophageal perforation. At present, there is no consensus for the management of delayed esophageal perforations. The encouraging results from this study can provide clinicians with an alternative, less invasive treatment approach for a challenging clinical problem which has traditionally carried a high rate of morbidity and mortality.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1316/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1316/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1316/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1316/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Chang Gung Memorial Hospital (No. 202100306B0) and informed consent was waived for this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Teh E, Edwards J, Duffy J, et al. Boerhaave's syndrome: a review of management and outcome. Interact Cardiovasc Thorac Surg 2007;6:640-3. [Crossref] [PubMed]
- Shaker H, Elsayed H, Whittle I, et al. The influence of the 'golden 24-h rule' on the prognosis of oesophageal perforation in the modern era. Eur J Cardiothorac Surg 2010;38:216-22. [Crossref] [PubMed]
- Muir AD, White J, McGuigan JA, et al. Treatment and outcomes of oesophageal perforation in a tertiary referral centre. Eur J Cardiothorac Surg 2003;23:799-804; discussion 804. [Crossref] [PubMed]
- Fischer A, Thomusch O, Benz S, et al. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg 2006;81:467-72. [Crossref] [PubMed]
- Hindy P, Hong J, Lam-Tsai Y, et al. A comprehensive review of esophageal stents. Gastroenterol Hepatol (N Y) 2012;8:526-34. [PubMed]
- Spaander MC, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:939-48. [Crossref] [PubMed]
- Siersema PD, Homs MY, Haringsma J, et al. Use of large-diameter metallic stents to seal traumatic nonmalignant perforations of the esophagus. Gastrointest Endosc 2003;58:356-61. [Crossref] [PubMed]
- Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal stent placement for the treatment of iatrogenic intrathoracic esophageal perforation. Ann Thorac Surg 2007;83:2003-7; discussion 2007-8. [Crossref] [PubMed]
- Freeman RK, Van Woerkom JM, Vyverberg A, et al. Esophageal stent placement for the treatment of spontaneous esophageal perforations. Ann Thorac Surg 2009;88:194-8. [Crossref] [PubMed]
- D'Cunha J, Rueth NM, Groth SS, et al. Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg 2011;142:39-46.e1. [Crossref] [PubMed]
- Koivukangas V, Biancari F, Meriläinen S, et al. Esophageal stenting for spontaneous esophageal perforation. J Trauma Acute Care Surg 2012;73:1011-3. [Crossref] [PubMed]
- Sharma P, Kozarek RPractice Parameters Committee of American College of Gastroenterology. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol 2010;105:258-73; quiz 274. [Crossref] [PubMed]
- van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011;33:1292-301. [Crossref] [PubMed]
- Dasari BV, Neely D, Kennedy A, et al. The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 2014;259:852-60. [Crossref] [PubMed]
- van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterol 2012;12:19. [Crossref] [PubMed]
- Jang S, Parsi M, Collins J, et al. Predictors of esophageal self-expandable metal stent migration: An academic center study. Gastrointestinal Intervention 2016;5:72-9. [Crossref]
- Karatepe O, Acet E, Altiok M, et al. Esophageal stent migration can lead to intestinal obstruction. N Am J Med Sci 2009;1:63-5. [PubMed]
- Khan S, George N, Tharian B. Extraluminal migration of an esophageal metal stent causing splenic injury. Endoscopy 2016;48:E326. [Crossref] [PubMed]
- Schweigert M, Sousa HS, Solymosi N, et al. Spotlight on esophageal perforation: A multinational study using the Pittsburgh esophageal perforation severity scoring system. J Thorac Cardiovasc Surg 2016;151:1002-9. [Crossref] [PubMed]
- Dickinson KJ, Blackmon SH. Endoscopic Techniques for the Management of Esophageal Perforation. Oper Tech Thorac Cardiovasc Surg 2015;20:251-78. [Crossref]
- Bick BL, Song LM, Buttar NS, et al. Stent-associated esophagorespiratory fistulas: incidence and risk factors. Gastrointest Endosc 2013;77:181-9. [Crossref] [PubMed]
- Hirdes MM, Vleggaar FP, Van der Linde K, et al. Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy 2011;43:156-9. [Crossref] [PubMed]
- van Halsema EE, Wong Kee Song LM, Baron TH, et al. Safety of endoscopic removal of self-expandable stents after treatment of benign esophageal diseases. Gastrointest Endosc 2013;77:18-28. [Crossref] [PubMed]
- Cameron JL, Kieffer RF, Hendrix TR, et al. Selective nonoperative management of contained intrathoracic esophageal disruptions. Ann Thorac Surg 1979;27:404-8. [Crossref] [PubMed]
- Yamashita R, Takeno S, Yamana I, et al. Successful Treatment of Septic Shock due to Spontaneous Esophageal Perforation 96 Hours after Onset by Drainage and Enteral Nutrition. Case Rep Gastroenterol 2014;8:387-92. [Crossref] [PubMed]
- Wigley C, Athanasiou A, Bhatti A, et al. Does the Pittsburgh Severity Score predict outcome in esophageal perforation? Dis Esophagus 2019;32. [Crossref] [PubMed]
- Jung CFM, Müller-Dornieden A, Gaedcke J, et al. Impact of Endoscopic Vacuum Therapy with Low Negative Pressure for Esophageal Perforations and Postoperative Anastomotic Esophageal Leaks. Digestion 2021;102:469-79. [Crossref] [PubMed]
- Richter F, Hendricks A, Schniewind B, et al. Eso-Sponge® for anastomotic leakage after oesophageal resection or perforation: outcomes from a national, prospective multicentre registry. BJS Open 2022;6:zrac030. [Crossref] [PubMed]


