
Preoperative umeclidinium/vilanterol or tiotropium improves postoperative FEV1 in lung cancer patients with comorbid untreated chronic obstructive pulmonary disease
Highlight box
Key findings
• Patients with lung cancer with untreated COPD were actively treated for COPD. In the COPD intervention group, the measured FEV1 value 3 months postoperatively was similar to that before the operation (−0.05 vs. −0.25 mL, P=0.0026) and was significantly higher than the predicted value (+0.33 vs. +0.04 mL, P<0.0001).
What is known and what is new?
• COPD is frequently diagnosed incidentally in patients with lung cancer; however, treatment for COPD may not be performed.
• In patients with lung cancer with untreated COPD, the active preoperative intervention improved respiratory function, expanded treatment options, and maintained respiratory function to the degree that exceeded preoperative predictions.
What is the implication, and what should change now?
• In patients with lung cancer with untreated COPD, the active preoperative intervention, particularly UMEC/VI, was more effective.
Introduction
Chronic obstructive pulmonary disease (COPD) is closely associated with predictors of lung cancer, its prognostic factors, and risk factors for perioperative complications (1-5). COPD is often diagnosed incidentally during preoperative pulmonary function tests in patients with lung cancer (1,2,6), but treatment for COPD may not be performed to a sufficient degree in case of asymptomatic patients. Untreated COPD in patients with lung cancer carries substantial risks, including missing standard treatment opportunities, an increased risk of postoperative complications, and worsened postoperative respiratory symptomology. Appropriate preoperative interventions for COPD may decrease these risks. There have been some reports regarding COPD treatment and the efficacy of minimally invasive surgery in this regard (7-10). However, the significance of preoperative treatment has not been fully evaluated in patients with COPD who are undergoing lung resection (11-14). In addition, although some lung resection guidelines recommend evaluating the diffusing capacity of the lungs for carbon monoxide and conducting exercise stress tests (15), treatment regimens may be selected without conducting these evaluations.
This study aimed to verify the frequency of preoperative interventions in lung cancer patients with COPD presenting at our medical center and to examine the effects of preoperative interventions in lung cancer patients with untreated COPD. We evaluated preoperative interventions using tiotropium (TIO) as well as umeclidinium/vilanterol (UMEC/VI) as a combination inhaler therapy, and included a greater number of patients than in previous studies on this topic. This article was presented in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1704/rc).
Methods
Study design and patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Joetsu General Hospital (Niigata, Japan; J-182). The requirement for informed consent was waived due to the study’s retrospective design. We included all lung cancer patients with COPD admitted to the Joetsu General Hospital and Toyama University Hospital (Toyama, Japan) for elective surgery between November 2011 and May 2018. In this study, the COPD was defined by a history of exposure to risk factors including secondhand smoking, and spirometry findings, despite of any symptoms. Spirometry is required to make the diagnosis in this clinical context; the presence of a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity <0.70 confirms the presence of persistent airflow limitation and thus of COPD in patients with appropriate symptoms and significant exposure to noxious stimuli, and exclusion of other diseases causing obstructive pulmonary disorders from differential diagnosis (7). Naturally, patients with or suspected bronchial obstruction by tumor were excluded. If characteristics of asthma were observed, the patient was diagnosed with asthma and COPD overlap (ACO) according to the Global Initiative for Asthma and Global Initiative for Chronic Obstructive Lung Disease guidelines (16). In this study, ACO was defined when a patients diagnosed as COPD and had some asthma features; exacerbations may be more common than in COPD but are reduced by treatment; progression is usual and treatment needs are high; frequently a history of doctor-diagnosed asthma, allergies and a family history of asthma; respiratory symptoms including exertional dyspnea are persistent but variability may be prominent; may have had symptoms in childhood or early adulthood.
The study exclusion criteria were as follows: asthma alone; undergoing partial lung resection, pneumonectomy, or emergency surgery; with or suspected bronchial obstruction by tumor; age ≤19 years; and treatment for COPD. Patients with ACO were not excluded from the present study. Patients with missing data were excluded from the analyses.
COPD treatments
The COPD treatment policy at our medical centers was as follows. No interventions were administered to patients between November 2011 to March 2013, whereas a therapeutic drug was prospectively prescribed starting in April 2013. Any bronchodilators were not used in the untreated group. TIO was later administered as inhalation therapy from April 2013 to September 2014 and UMEC/VI from October 2014 to June 2017. These drugs were each administered starting from 2 weeks before surgery and were continued until 3 months after surgery. An expectorant was used in all cases.
Preoperative tests
Simple chest radiography, contrast-enhanced computed tomography, blood tests, urine tests, electrocardiograms, and pulmonary function tests were performed in all cases. Spirometry was evaluated after bronchodilator administration. In cases with lung cancer, cerebral magnetic resonance imaging and positron emission tomography were also performed. Patients aged >80 years with one or more risk factors for coronary artery disease underwent preoperative cardiac echography and cardiac stress testing.
Surgical strategy
Radical lobectomy and node dissections were performed in patients with an FEV1 of ≥1.5 L. If a well-differentiated lung adenocarcinoma of ≤2 cm with a tumor stage of cT1aN0M0 or less was diagnosed, segmentectomy was performed, even with an FEV1 of ≥1.5 L. If FEV1 was <1.5 L, patients were informed of the potential perioperative complications and local recurrence risks. Radical lobectomy was performed if the patient actively selected this procedure or if the surgeon deemed it necessary. Segmentectomy was performed if the patient was reluctant to undergo lobectomy or if the risk of perioperative complications was very high. In addition, preoperative pulmonary training using TRI-BALLTM (Medtronic, Dublin, Ireland) as an exercise device was performed in all surgical patients. No postoperative rehabilitation was performed. Radiation therapy was administered at the patients’ request or if the surgeon deemed radiation therapy more appropriate than surgery. All patients who smoked were advised to stop smoking and were provided treatment for smoking cessation regardless of the administered COPD treatment.
Surgical procedure
General anesthesia was maintained using single-lung ventilation with a double-lumen endotracheal tube. Patients were placed in the lateral decubitus position. The surgical approach involved video-assisted thoracoscopic surgery (VATS). Patients underwent four-port VATS with three 5-mm ports and one 10-mm port for lobectomy and segmentectomy. A thoracoscope was used with a 30° 5- or 10-mm camera. During specimen extraction, a one-port incision was extended to approximately 3 cm.
Pain management
For standard pain management, all patients received epidural analgesia and oral drugs. Patients received loxoprofen 180 mg/day from the first postoperative day and pregabalin 25 mg twice daily from the second postoperative day, for a total of 3 months; acetaminophen 1,200 mg/day was prescribed to patients with an estimated glomerular filtration rate of <50 mL/min. Medication was continued until a pain-free status was achieved, as in a previous report (17).
Variables and assessments
The following patient characteristics, surgical characteristics, and follow-up parameters were recorded from the preoperative period until 3 months after surgery: age, sex, past medical history, steroid exposure, smoking history, body mass index, respiratory function as measured by spirometry, lung cancer and COPD diagnosis, disease side, tumor size, current lung disease other than lung cancer (emphysema, interstitial pneumonia), procedure type (partial resection, segmentectomy, lobectomy), number of resected lung segments, surgical approach (VATS, thoracotomy), intraoperative bleeding, operative time, chest tube duration, complications (e.g., prolonged air leak defined as an air leak lasting for >5 days), and inhalation therapy and its side effects. Complications were defined as any deviation from the normal postoperative course and were graded according to the Clavien-Dindo classification.
Pulmonary function tests were performed before administering the prescription, 2 weeks after prescription, and 3 months after surgery. The following formula was used to derive postoperative predicted respiratory function: predicted postoperative FEV1 = preoperative FEV1 × (19 − segments to be removed/19) (18). The side effects of inhalation therapy were measured in cases of thirst, urination difficulty, eye symptoms, palpitation, arrhythmia, headache, nausea, vomiting, and low blood pressure, when there was no cause other than inhalation therapy.
Data management and statistical analysis
This was a retrospective study using data collected at Toyama University Hospital and Joetsu General Hospital. Sample size was calculated based on previous studies (1,2,6,11-14). According to previous findings, we expected that 35% of lung cancer patients presenting at our medical center would have coexisting COPD. In consideration of a targeted statistical power of 80% and two-sided statistical significance level of 5%, the necessary sample size was estimated as 70 patients. Expecting a dropout rate of 10% and given a lack of data on the sample size necessary for the intervention group, we initially aimed to recruit 116 patients.
For the univariate analysis, intergroup differences were evaluated using the non-parametric Wilcoxon rank-sum test. The χ2 or Fisher’s exact test was used to compare categorical variables as appropriate. Statistical significance was defined as a two-sided P value of <0.05. Continuous variables are presented as mean ± standard deviation for normally distributed data, and as median with interquartile range for non-normally distributed data. Categorical variables are presented as sample size and percentage [n (%)]. All statistical analyses were performed using JMP version 15.0 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 387 patients visited our clinic for lung cancer surgery during the study period, of whom 133 (34.4%) had coexisting COPD (Figure 1). Of these, 10 (7.5%) had already been treated for COPD, and the remaining 123 (92.5%) had not been treated; 111 of 123 (90.2%) of these patients had no COPD symptoms. Moreover, of the 123 patients, 31 were excluded from the present study because they underwent partial lung resection. A total of 92 patients were enrolled; 31 patients were classified into the untreated group, and 61 were classified into the intervention group. Of the 61 patients, seven (11.5%) were diagnosed with ACO. In the intervention group, 45 (73.8%) patients were prescribed the UMEC/VI intervention and 16 (26.2%) received TIO.
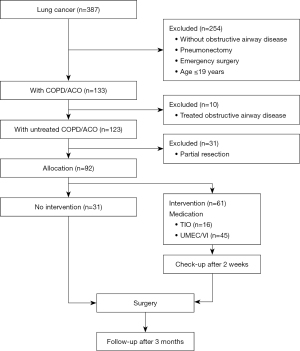
The intervention group showed a greater increase in FEV1 than the untreated group (FEV1: 120 vs. 0 mL, P=0.014). In the intervention group, the UMEC/VI group showed a greater increase in FEV1 than the TIO group (FEV1: 160 vs. 7 mL, P=0.0005). Fifteen of the 61 patients (24.6%) in the intervention group had an FEV1 of <1.5 L before being administered the prescription. However, in nine of these 15 patients (60.0%) in the intervention group, FEV1 increased to ≥1.5 L after intervention; all of these patients underwent radical lobectomy. Eight of the nine patients (88.9%) were prescribed the UMEC/VI. Three of the six patients (50.0%) in the FEV1 of <1.5 L after the prescription were received TIO.
Untreated group vs. intervention group in all anatomical lung resection patients
There were no significant differences in preoperative background factors between the intervention group and untreated group (Table 1). The intervention group had significantly less bleeding (70 vs. 120 mL, P=0.0054), a shorter tube drainage period (1 vs. 3 days, P<0.0001), and a shorter postoperative hospital stay (8 vs. 10 days, P=0.021) than the untreated group. No significant differences were observed in other postoperative factors between the groups. Moreover, no significant differences in postoperative factors were observed between the group with a preintervention FEV1 of ≥1.5 L and the group with a postintervention FEV1 of ≥1.5 L.
Table 1
| Characteristics | Untreated (n=31) | Intervention (n=61) | P value |
|---|---|---|---|
| Age (year), median [IQR] | 72 [64–79] | 70 [67–78] | 0.70 |
| Sex, male, n (%) | 27 (87.1) | 51 (83.6) | 0.77 |
| BMI, median [IQR] | 22.7 [20.7–24.9] | 22.5 [20.5–24.3] | 0.52 |
| Hypertension, n (%) | 21 (67.7) | 29 (47.5) | 0.079 |
| Hyperlipidemia, n (%) | 3 (9.7) | 15 (24.6) | 0.10 |
| Hyperuricemia, n (%) | 1 (3.2) | 9 (14.8) | 0.16 |
| Diabetes, n (%) | 6 (19.4) | 12 (19.7) | 0.60 |
| Steroid exposure, n (%) | 1 (3.2) | 4 (6.6) | 0.66 |
| Smoking history, n (%) | 26 (83.9) | 53 (86.9) | 0.76 |
| Brinkman index, median [IQR] | 875 [724–1,350] | 970 [780–1,124] | 0.95 |
| Comorbidities, n (%) | |||
| Osteoporosis | 2 (6.7) | 3 (4.9) | 0.80 |
| Gastroesophageal reflux | 4 (13.3) | 4 (6.6) | 0.43 |
| Cardiovascular diseases | 6 (20.0) | 15 (24.6) | 0.79 |
| Anxiety and depression | 10 (33.3) | 14 (23.0) | 0.32 |
| Interstitial pneumonia, n (%) | 4 (12.9) | 9 (14.8) | 0.54 |
| GOLD classification, n (%) | 0.097 | ||
| 2 | 24 (77.4) | 56 (91.8) | |
| 3 | 7 (22.6) | 5 (8.2) | |
| Spirometry, median [IQR] | |||
| FVC (L) | 3.51 [3.04–3.91] | 3.18 [2.69–3.76] | 0.10 |
| %FVC (%) | 105.3 [91.0–116.5] | 100.2 [87.7–111.6] | 0.19 |
| FEV1/FVC (%) | 63.0 [54.0–67.3] | 64.5 [59.2–67.7] | 0.34 |
| FEV1 (L) | 2.19 [1.58–2.67] | 1.96 [1.58–2.40] | 0.30 |
| Diseased side, right, n (%) | 18 (58.1) | 35 (57.4) | 0.61 |
| Tumor size (mm), median [IQR] | 30 [19–36] | 21 [16–32] | 0.11 |
| Clinical N1/2, n (%) | 7 (22.6) | 11 (18.6) | 0.78 |
| Location, upper segments, n (%) | 22 (71.0) | 37 (60.7) | 0.44 |
| VATS, n (%) | 24 (77.4) | 55 (90.2) | 0.12 |
| Procedure, n (%) | 0.077 | ||
| Segmentectomy | 12 (38.7) | 12 (19.7) | |
| Lobectomy | 19 (61.3) | 49 (80.3) | |
| Number of resected segments (n), median [IQR] | 3 [3–4] | 3 [3–5] | 0.26 |
| Intraoperative bleeding (mL), median [IQR] | 120 [60–330] | 70 [13–150] | 0.0054 |
| Operative time (min), median [IQR] | 199 [159–240] | 198 [173–248] | 0.81 |
| Chest tube duration (day), median [IQR] | 3 [2–6] | 1 [1–3] | <0.0001 |
| Complications, n (%) | |||
| Total | 13 (41.9) | 20 (32.8) | 0.49 |
| Prolonged air leak | 6 (19.4) | 7 (11.5) | 0.35 |
| Pneumonia | 1 (3.2) | 4 (6.6) | 0.66 |
| Atelectasis | 1 (3.2) | 1 (1.6) | 0.89 |
| Arrhythmia | 5 (16.1) | 8 (13.1) | 0.76 |
| Delirium | 2 (6.5) | 2 (3.3) | 0.60 |
| Postoperative bleeding | 1 (3.2) | 0 (0) | 0.34 |
| Others | 0 (0) | 1 (1.7) | 0.66 |
| Postoperative hospitalization (day), median [IQR] | 10 [8–13] | 8 [6–10] | 0.021 |
COPD, chronic obstructive pulmonary disease; ACO, Asthma-COPD overlap; IQR, interquartile range; BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; N, lymph nodes; VATS, video-assisted thoracoscopic surgery.
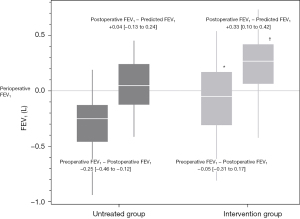
FEV1 at 3 months after surgery in the intervention group was almost the same as preoperative FEV1 (−0.05 vs. −0.25 mL, P=0.0026), unlike in the untreated group. FEV1 after 3 months in the untreated group was similar to the preoperative predicted value, whereas that of the intervention group was significantly higher than the predicted value (+0.33 vs. +0.04 mL, P<0.0001) (Table 2, Figure 2). No obvious side effects were observed in reference to the therapeutic agents administered in the intervention group. None of the patients forgot to use their inhaler or were otherwise non-compliant with the intervention.
Table 2
| Parameter | FEV1 (L), median [IQR] | P value | |
|---|---|---|---|
| Untreated (n=31) | Intervention (n=61) | ||
| Before surgery | |||
| FEV1 | 2.19 [1.58–2.67] | 1.96 [1.58–2.40] | 0.30 |
| Postint FEV1 | 2.18 [1.71–2.51] | ||
| Postint − Preint FEV1 | 0.12 [0.06–0.26] | ||
| Predicted postop FEV1 | 1.80 [1.32–2.10] | 1.53 [1.22–1.91] | 0.18 |
| Predicted postop after postint FEV1 | 1.62 [1.37–2.05] | ||
| After surgery | |||
| Postop FEV1 | 1.73 [1.38–2.09] | 1.91 [1.59–2.26] | 0.11 |
| Postop − Preop FEV1 | −0.25 [−0.46 to −0.12] | −0.05 [−0.31 to 0.17] | 0.0026 |
| Postop − Predicted FEV1 | 0.04 [−0.13 to 0.24] | 0.33 [0.10–0.54] | <0.0001 |
| Postop − Predicted after postint FEV1 | 0.27 [0.06–0.42] | ||
COPD, chronic obstructive pulmonary disease; ACO, Asthma-COPD overlap; FEV1, forced expiratory volume in 1 second; IQR, interquartile range; int, intervention; op, operative.
Untreated group vs. intervention group in lobectomy patients
Among the patients who underwent lobectomy, the intervention group had significantly less gastroesophageal reflux (6.1% vs. 26.3%, P=0.034) and intraoperative bleeding (75 vs. 145 mL, P=0.016) than the untreated group (Table S1). Additionally, the intervention group had a shorter tube drainage period (1 vs. 3 days, P=0.0004) and postoperative hospital stay (8 vs. 10 days, P=0.049) than the untreated group.
Unlike in the untreated group, the FEV1 at 3 months postoperatively in the intervention group was almost the same as the preoperative value (−0.07 vs. −0.21 mL, P=0.021). Furthermore, the FEV1 at 3 months postoperatively in the untreated group was similar to the preoperative predicted value, whereas that of the intervention group was significantly higher than the predicted value (+0.38 vs. +0.09 mL, P=0.0008) (Table S2).
Untreated group vs. intervention group in segmentectomy patients
There were no significant differences in perioperative factors between the intervention and untreated groups among patients who underwent segmentectomy (Table S3). Unlike in the untreated group, the FEV1 at 3 months postoperatively in the intervention group was the same as the preoperative value (0.00 vs. −0.29 mL, P=0.033). Furthermore, the FEV1 at 3 months postoperatively in the untreated group was similar to the preoperative predicted value, whereas that of the intervention group was significantly higher than the predicted value (+0.25 vs. +0.03 mL, P=0.036) (Table S4).
Intervention group: TIO vs. UMEC/VI
There were no significant differences in preoperative factors between patients administered TIO and UMEC/VI in the intervention group. The UMEC/VI subgroup showed a greater increase in FEV1 than the TIO subgroup (FEV1: 160 vs. 7 mL, P=0.0005) and fewer postoperative complications (22.2% vs. 62.5%, P=0.0053) (Table S5). Furthermore, there were no significant differences in FEV1 between the groups at 3 months postoperatively (Table S6).
Discussion
In this study, lung cancer patients with untreated COPD were actively treated for COPD. In the COPD intervention group, respiratory function was found to improve 2 weeks after inhalation therapy (especially with the use of UMEC/VI). The measured FEV1 value 3 months after surgery was similar to that before the operation (−0.05 vs. −0.25 mL, P=0.0026) and was significantly higher than the predicted value (+0.33 vs. +0.04 mL, P<0.0001). Similar results were obtained for each subgroup analysis of patients who underwent lobectomy and segmentectomy. Hence, we conclude that active preoperative intervention for COPD can contribute to maintaining respiratory function. In addition, we found that the evaluated COPD interventions enabled radical and safe anatomical lung resection without missing treatment opportunities in patients with poor respiratory function. In particular, UMEC/VI was found to be more effective (FEV1 improvement: 160 vs. 7 mL, P=0.0005) and had fewer postoperative complications (22.2 vs. 62.5%, P=0.0053) than TIO. However, there were no significant differences in postoperative FEV1 between the groups.
In COPD, respiratory function worsens gradually, and it takes time for symptoms to appear and for the diagnosis to be determined. In Japan, according to a report, 10.9% of people aged >40 years are reported to have obstructive disorders, 9.4% are diagnosed with COPD, and 90.6% are undiagnosed and untreated COPD patients (6). The relationship between perioperative outcomes and COPD is considered poor not only for lung cancer but also for diseases of other organs (19-21). This study was a relatively short-term (i.e., 3-month) study, and the long-term prognosis in regard to this preoperative intervention is unknown. In contrast, the effects of COPD inhalation therapy itself are well known, and it seems to be of great significance to introduce this intervention preoperatively, even in asymptomatic COPD patients. However, there are negative opinions in the medical community in regard to the costs of interventions for asymptomatic COPD patients. We note that, in this study, if a patient did not wish to continue treatment for COPD, the prescription was discontinued after the 3-month study period. This study included patients who were asymptomatic and undiagnosed before surgery. Therefore, the history of acute exacerbation could not be clearly determined. This study examined steroid therapy, which may be associated with acute exacerbation and ACO pathology; however, the number of patients on steroid therapy was small, and no significant difference was observed.
COPD is also a known risk factor for various adverse outcomes in the perioperative period, and there have been various research reports evaluating this topic. Pulmonary resection in patients with COPD is not only associated with postoperative respiratory complications (atelectasis, persistent air leak, pneumonia) but is also associated with arrhythmia (3-5). However, the frequency of complications is also influenced by the surgical approach. VATS is associated with a lower risk of complications than thoracotomy (7-10). In this study, there was no significant difference in postoperative complications between the intervention and untreated groups, although the intervention group showed a trend toward fewer complications (32.8% vs. 41.9%, P=0.49). Postoperative complications, especially, were reduced in the UMEC/VI subgroup. The amount of bleeding, drainage period, and length of postoperative hospital stay were superior in the intervention group, but it cannot be concluded that this was due to the intervention because of complicating factors such as the comparatively long study period. However, previous reports have shown with certainty that preoperative interventions for untreated COPD help maintain good respiratory function (11-14). In addition, volume reduction effects may be expected in regard to pulmonary emphysema-type COPD and upper lobectomy (22); however, expectations are low for surgical procedures other than upper lobectomy. This study evaluated lung volume reduction effects in cases involving excision of the upper lobe, segment six lesions, and other excisions. Nevertheless, the intervention was considered effective. Although no difference was observed in the FEV1 at 3 months postoperatively, UMEC/VI may be a more effective intervention than TIO.
Among diseases that require differentiation from COPD, it is difficult to distinguish COPD from asthma, and ACO is often diagnosed as a complicated disease (16). COPD, asthma, and ACO are each treated mainly with airway dilators. Specifically, three drugs are implemented in treating these conditions: long-acting muscarinic antagonists (LAMAs), long-acting beta agonists (LABAs), and inhaled corticosteroids (ICSs). In this study, COPD medications were selected according to disease severity and combined with asthma medications. We assumed that asymptomatic patients may forget to use their drugs. Therefore, we selected drugs that only had to be inhaled once a day. UMEC/VI, as the combination drug, became the first choice among prescribing physicians after gaining approval for clinical use in Japan, because this drug shows a significantly better effect than TIO (23). In this study, the patients were highly conscientious regarding their prescribed treatment, and there were no cases in which patients forgot to use their inhaler (likely because these patients were planning to undergo surgery). Since we enrolled many elderly male patients with lung cancer, there were concerns about commonly occurring side effects due to LAMAs, particularly dysuria, but none of the patients complained of worsening dysuria.
Moreover, the combination of ICSs and LABAs is recommended for asthma; however, ICS is associated with a risk of complications due to pneumonia (24). It may therefore be safer not to add ICSs during the perioperative period. However, in patients with low pulmonary function, ICSs can be added in order to enhance respiratory function. In the future, we will investigate the long-term prognosis of lung cancer patients who have undergone COPD treatment intervention, evaluate symptoms, and examine the effects and complications of triple inhalation therapy using LAMAs, LAVAs, and ICSs.
Limitations
The limitations of this study include its retrospective design, involving two institutions, selection of inhaled drugs, diagnosis, and slight lung volume reduction effect. In addition, this work evaluated both TIO and UMEC/VI as interventions because of different hospital-level protocols specified at different time periods during the course of the study. Many other inhaled drugs and inhalation devices are used in clinical practice, and many studies have reported on each of these interventions. Other drugs or inhalation devices may be more effective. However, the purpose of this study was not to evaluate differences between inhaled drugs or devices, but instead to examine the specific effects of preoperative intervention. In cases where upper lobectomy or S6 resection was performed, it is undeniable that not only inhaled drug effects but also lung volume reducing effects were more likely to be observed. This observation was further evaluated in consideration of the resected areas and numbers of resections. The diagnosis was evaluated by spirometry after bronchodilator administration; however, some patients were nonsmokers, thereby making it difficult to rule out asthma (25).
Conclusions
In lung cancer patients with untreated COPD and ACO, the evaluated preoperative interventions not only improved respiratory function but also expanded treatment options and maintained respiratory function to a degree that exceeded preoperative predictions. This may have contributed to reduction in the risk of perioperative complications and to the minimization of subjective symptoms.
Acknowledgments
This study was presented at the 26th Asian Society for Cardiovascular and Thoracic Surgery Meeting hosted in Moscow in 2018. We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The author has completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1704/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1704/dss
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1704/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Joetsu General Hospital (Niigata, Japan; J-182). The requirement for informed consent was waived due to the study’s retrospective design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Radin A, Cote C. Primary care of the patient with chronic obstructive pulmonary disease-part 1: frontline prevention and early diagnosis. Am J Med 2008;121:S3-12. [Crossref] [PubMed]
- Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 2005;128:2099-107. [Crossref] [PubMed]
- Bugge A, Lund MB, Brunborg C, et al. Survival After Surgical Resection for Lung Cancer in Patients With Chronic Obstructive Pulmonary Disease. Ann Thorac Surg 2016;101:2125-31. [Crossref] [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002;37:95-101. [Crossref] [PubMed]
- Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology 2004;9:458-65. [Crossref] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2022 Report).
- Berry MF, Villamizar-Ortiz NR, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51; discussion 1051-2. [Crossref] [PubMed]
- Ceppa DP, Kosinski AS, Berry MF, et al. Thoracoscopic lobectomy has increasing benefit in patients with pulmonary function: a society of thoracic surgeons database analysis. Ann Surg 2012;256:487-93. [Crossref] [PubMed]
- Oparka J, Yan TD, Ryan E, et al. Does video-assisted thoracic surgery provide a safe alternative to conventional techniques in patients with limited pulmonary function who are otherwise suitable for lung resection? Interact Cardiovasc Thorac Surg 2013;17:159-62. [Crossref] [PubMed]
- Suzuki H, Sekine Y, Yoshida S, et al. Efficacy of perioperative administration of long-acting bronchodilator on postoperative pulmonary function and quality of life in lung cancer patients with chronic obstructive pulmonary disease. Preliminary results of a randomized control study. Surg Today 2010;40:923-30. [Crossref] [PubMed]
- Ueda K, Tanaka T, Hayashi M, et al. Role of inhaled tiotropium on the perioperative outcomes of patients with lung cancer and chronic obstructive pulmonary disease. Thorac Cardiovasc Surg 2010;58:38-42. [Crossref] [PubMed]
- Kobayashi S, Suzuki S, Niikawa H, et al. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology 2009;14:675-9. [Crossref] [PubMed]
- Bolukbas S, Eberlein M, Ecknhoff J, et al. Short-term effects of inhalative tiotropium/formoterol/budenoside versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer. Eur J Cardiovasc Surg 2011;39:995-1000. [Crossref] [PubMed]
- Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- A joint project of GINA and GOLD. Diagnosis and initial treatment of asthma, COPD and asthma - COPD overlap (updated April 2017).
- Homma T, Doki Y, Yamamoto Y, et al. Efficacy of 50 mg pregabalin for prevention of postoperative neuropathic pain after video-assisted thoracoscopic surgery and thoracotomy: a 3-month prospective randomized controlled study. J Thorac Dis 2019;11:694-701. [Crossref] [PubMed]
- Ali MK, Mountain CF, Ewer MS, et al. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980;77:337-42. [Crossref] [PubMed]
- Cingoz F, Oz BS, Arslan G, et al. Is chronic obstructive pulmonary disease a risk factor for epistaxis after coronary artery bypass graft surgery? Cardiovasc J Afr 2014;25:279-81. [Crossref] [PubMed]
- Martin CT, Gao Y, Pugely AJ, et al. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg 2013;22:1667-1675.e1. [Crossref] [PubMed]
- Goto T, Tsugawa Y, Faridi MK, et al. Reduced risk of acute exacerbation of COPD after bariatric surgery. A self-controlled case series study. Chest 2018;153:611-7. [Crossref] [PubMed]
- Lim E, Sousa I, Shah PL, et al. Lung Volume Reduction Surgery: Reinterpreted With Longitudinal Data Analyses Methodology. Ann Thorac Surg 2020;109:1496-501. [Crossref] [PubMed]
- Maleki-Yazdi MR, Kaelin T, Richard N, et al. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med 2014;108:1752-60. [Crossref] [PubMed]
- Suissa S, Coulombe J, Ernst P. Discontinuation of Inhaled Corticosteroids in COPD and the Risk Reduction of Pneumonia. Chest 2015;148:1177-83. [Crossref] [PubMed]
- Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers. Results from the population-based burden of obstructive lung disease study. Chest 2011;139:752-63. [Crossref] [PubMed]


