
Impact of delayed transition off intravenous vasoactive agents for aortic dissection on intensive care unit length of stay
Highlight box
Key findings
• In patients with acute aortic dissection, transitioning from vasoactive infusions to enteral antihypertensives within 72 hours may be associated with a shorter intensive care unit stay, without an increase in hypotension.
What is known and what is new?
• Urgent hemodynamic management is a hallmark of guideline-directed therapy in patients with acute aortic dissection. Commonly, this requires the initiation of continuous antihypertensive infusions, but there is limited guidance available for when and how to transition off intravenous medications to enteral agents. This study will compare the impact of rapid versus slow transition from intravenous to enteral antihypertensive medications on intensive care unit length of stay.
What is the implication, and what should change now?
• This study supports that patients with acute aortic dissection can be safely transitioned from vasoactive infusions to enteral antihypertensive agents within 72 hours. Using enteral agents to wean antihypertensive infusions may be a promising strategy to reduce intensive care unit length of stay.
Introduction
An aortic dissection describes an acute condition where a tear in the intimal lining of the aorta allows blood to flow through the tear between the layers of the aortic wall into a false lumen, further separating the aortic layers. Aortic dissection is a medical emergency. Complications include cardiac tamponade, aortic rupture, and circulatory failure in addition to consequences that arise from impaired perfusion to vital organs.
Acute management of aortic dissection involves rapid control of blood pressure (BP) and heart rate (HR) in order to decrease aortic shear stress and minimize the risk of dissection extension and aortic rupture. The corner stone of management is initiation of an intravenous (IV) beta blocker with or without a vasodilator to achieve hemodynamic goals of systolic BP <120 mmHg and HR <60 to minimize aortic wall stress (1). Clinical guidelines are clear in the endorsement of rapid initiation of IV agents for BP and HR control to the lowest doses that will sustain adequate end-organ perfusion, but when to transition to and how to titrate enteral or per os (PO) anti-hypertensive medications is less clear (1,2). As a result, patients can remain in the intensive care unit (ICU) for close hemodynamic monitoring required for continuous IV medications, even if these patients are hemodynamically stable with consistent infusion requirements who are otherwise ready for floor transfer. Occasionally, this requirement is the only indication for ICU level of care. Deferred conversion to PO agents in these patients may lead to extended ICU stays, additional costs, and inefficient resource utilization.
The challenge of delayed transitioning from IV vasoactive infusions resulting in extended ICU length of stay has been described in patients with hypertensive intracerebral hemorrhages (3,4). Newer literature reports this issue in the aortic dissection population (5).
The purpose of this study is to compare the impact of rapid (within 72 hours of IV vasoactive infusion initiation) versus slow (greater than 72 hours of IV vasoactive infusion initiation) transition from IV to PO antihypertensive medications on ICU length of stay. We hypothesize that patients in the rapid group would be discharged from the ICU more quickly than those in the slow group. We present the following article in accordance with the TREND reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1274/rc).
Methods
This was a retrospective, single-center study of adult patients admitted to a single quaternary academic medical center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of University of California, San Francisco (UCSF), Laurel Heights Committee (Registration No. 00003471) and individual consent for this retrospective analysis was waived (IRB 20-31817, Reference #291091). Limited data were collected from review of medical records of patients who were admitted with an aortic dissection in recent years. The data were deidentified and available only to researchers. Results of this research will not affect the clinical care of the patients, who were already discharged from the hospital.
The Vizient® Clinical Data Base (Vizient, Irving, Texas) is a healthcare analytics platform that seeks to share outcomes data, collected from partnering academic medical centers. This database was used to generate a report that identified patients that were admitted to UCSF Health between January 2015 and November 2020 with a primary diagnosis of acute aortic dissection (ICD-10 code I71, I710, I7100, I7101, I7102, I7103, I711, I712, I713, I714, I715, I716, I718, I719).
The electronic medical record (EPIC, Verona, WI) was then reviewed to record patient demographic information for all study participants. All data were previously obtained in the course of standard patient care. Study data were collected and managed using REDCap electronic data capture tools hosted at UCSF.
Patients were eligible for inclusion in this study if they required IV antihypertensive medication infusions for a duration greater than 6 hours and had a diagnosis of aortic dissection. We selected this 6-hour time point to filter the list of eligible patients to exclude those who only transiently required IV antihypertensive infusions to meet hemodynamic goals and likely did not need oral medications to help wean these infusions. Studied vasoactive agents included esmolol, nicardipine, nitroprusside, nitroglycerin, clevidipine, or labetalol. Patients were excluded if they did not require enteral antihypertensive medications to meet hemodynamic goals prior to discharge from the ICU, if they expired prior to the initiation of enteral antihypertensive medications, or if they comprised vulnerable populations (including pregnant patients and prisoners).
Eligible patients were grouped based on the time required to complete a full transition from IV to PO antihypertensive medications. We opted to use the full transition or end of IV medications to determine patient groups, rather than initiation of PO medications, to evaluate the role of PO medications in helping to wean IV infusions. For the purposes of this study, patients who completed full transition within 72 hours from when the IV vasoactive infusion was initiated were considered the rapid group. Those who required greater than 72 hours were considered the slow group. We selected this 72-hour time point, since that was now the standard reported in the literature (5). A timeline of significant events during patient admission is depicted in Figure 1.
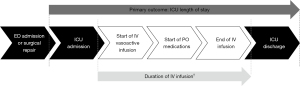
The primary endpoint was ICU length of stay in days. Secondary outcomes included hospital length of stay, duration of IV infusions, medication cost, incidence of hypotension (systolic BP <90 mmHg), and incidence of subsequent aortic events.
Evaluating the time to initiation of PO medications involved consideration of the time that enteral access was established. For patients who underwent surgical repair or were made “strict nil per os (NPO)” during their admission, enteral access was considered established after the first administration of enteral feedings or administration of any enteral medication (including non-antihypertensive agents). For the remainder of patients, the admission time was considered the time that enteral access was achieved.
Statistical analysis
Using an alpha of 0.05, our study had 80% power to detect a hypothesized standardized effect size of 0.7. Our study required 62 patients to detect a 7-day difference in ICU length of stay, as reported in previous literature (5). Our primary outcome was analyzed using the Wilcoxon rank-sum test, and secondary outcomes were evaluated using the Wilcoxon rank-sum test, independent t-test, or Chi-squared test. Normality was evaluated with the Shapiro-Wilk test. Statistical analyses were conducted using Stata/SE (version 15.2, College Station, Texas).
Results
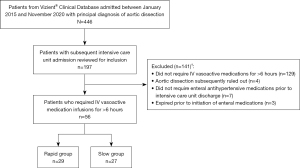
This study reviewed 197 patients to enroll 56 eligible patients, 29 of whom were transitioned in ≤72 hours (rapid group) and 27 of whom required >72 hours (slow group), as shown in Figure 2.
Baseline information
Baseline demographic information and clinical characteristics were similar between cohorts and are found in Table 1, though rapid group was older (63 vs. 56 years, P=0.08) and had proportionately more male patients (75.8% vs. 51.9%, P=0.09). The groups were balanced relative to Stanford classification of aortic dissection. There was a greater proportion of patients in the rapid transition group compared to the slow transition group that required surgical repair of their dissection (75.8% vs. 51.9%, P=0.67). There was also no difference in the total operating room time between the cohorts for those that required surgical repair (7.7 vs. 7.6 hours, P=0.91). Peak systolic BP and HR (within 6 hours of emergency department admission or peak reported at referring institution) were similar between the two cohorts (166 vs. 179 mmHg, P=0.10).
Table 1
| Characteristics | Slow (n=27) | Rapid (n=29) | P |
|---|---|---|---|
| Age (years), mean ± SD | 56±17 | 63±14 | 0.08 |
| Male sex, n (%) | 14 (51.9) | 22 (75.8) | 0.09 |
| Weight (kg), median [IQR] | 74.0 [65.8–90.0] | 74.0 [62.7–86.1] | 0.77 |
| History of connective tissue disorder, n (%) | 2 (7.4) | 2 (6.9) | 0.94 |
| History of previous known dissection, n (%) | 6 (22.2) | 9 (31.0) | 0.46 |
| Type A aortic dissection, n (%) | 13 (48.1) | 13 (44.8) | 0.60 |
| Required surgical repair of dissection, n (%) | 14 (51.9) | 22 (75.8) | 0.67 |
| Graft placement, n | 7 | 9 | |
| Endovascular repair, n | 3 | 7 | |
| Graft + aortic valve replacement, n | 2 | 1 | |
| Graft + aortic arch replacement, n | 2 | 5 | |
| Total operating room time (hours)†, mean ± SD | 7.7±3.1 | 7.6±2.5 | 0.91 |
| Peak systolic blood pressure (mmHg), mean ± SD | 166±32 | 179±28 | 0.10 |
| Peak heart rate (beats/min), mean ± SD | 76±15 | 76±13 | 0.85 |
| Primary team, n (%) | 0.60 | ||
| Cardiothoracic surgery | 9 (33.3) | 13 (44.8) | |
| Vascular surgery | 16 (59.3) | 15 (51.7) | |
| Cardiology | 2 (7.4) | 1 (3.4) | |
| Number of antihypertensive agents prior to admission, n (%) | 0.48 | ||
| 0 | 10 (37.0) | 8 (27.6) | |
| 1 | 8 (29.6) | 9 (31.0) | |
| 2 | 6 (22.2) | 7 (24.1) | |
| 3 | 3 (11.1) | 2 (6.9) | |
| 4 or more | 0 (0.0) | 3 (10.3) | |
| Type of medication used prior to admission, n (%) | |||
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 9 (33.3) | 10 (34.5) | 0.74 |
| Thiazide diuretic | 3 (11.1) | 3 (10.3) | 0.78 |
| Calcium channel blocker | 5 (18.5) | 9 (31.0) | 0.39 |
| Beta blocker | 10 (37.0) | 14 (48.3) | 0.62 |
| Alpha blocker | 3 (11.1) | 2 (6.9) | 0.46 |
| Direct vasodilator | 1 (3.7) | 3 (10.3) | 0.40 |
†, for patients requiring surgical repair: n=13 in slow group, n=22 in rapid group. SD, standard deviation; IQR, interquartile range.
A majority of patients (67.9%) had a history of hypertension and used antihypertensive agents prior to their admission. The most commonly used classes of agents were renin-angiotensin-aldosterone system (RAAS) inhibitors [including angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB)], beta blockers, and calcium channel blockers. Use of antihypertensive agent classes were similar between cohorts, as seen in Table 1.
Inpatient hemodynamic management
The most commonly used IV vasoactive agents were esmolol (60.7%), nicardipine (57.1%), and clevidipine (28.6%). The usage of IV agents was evenly distributed, though there was a notably higher usage of esmolol in the slow group compared to the rapid group (74.1% vs. 48.3%). The 72-hour mean doses and peak doses of the IV agents used are provided in Table 2 and did not differ significantly between groups. To transit from an IV to PO antihypertensive regimen, the most commonly ordered PO agents were amlodipine (62.5%), labetalol (67.9%), and metoprolol (42.9%). All hemodynamic management data are shown in Table 2.
Table 2
| Characteristicz | Slow (n=27) | Rapid (n=29) | P |
|---|---|---|---|
| IV vasoactive agent used, n (%) | – | ||
| Esmolol | 20 (74.1) | 14 (48.3) | |
| Nicardipine | 18 (66.7) | 14 (48.3) | |
| Clevidipine | 6 (22.2) | 10 (34.5) | |
| Labetalol | 7 (25.9) | 6 (20.7) | |
| Nitroglycerin | 3 (11.1) | 5 (17.2) | |
| Nitroprusside | 4 (14.8) | 1 (3.4) | |
| Mean dose of IV vasoactive at 72 hours, mean ± SD | |||
| Esmolol (μg/kg/min) | 175.0±73.4 | 144.0±66.9 | 0.31 |
| Nicardipine (mg/h) | 9.9±3.3 | 7.7±3.3 | 0.13 |
| Clevidipine (mg/h) | 9.4±3.5 | 8.9±5.5 | 0.86 |
| Labetalol (mg/min) | 2.8±1.6 | 2.1±1.4 | 0.50 |
| Nitroglycerin (μg/min) | 88±23 | 99±52 | 0.71 |
| Nitroprusside (μg/kg/min) | 1.5±0.8 | 1.0±0.0 | 0.59 |
| Oral agents used, n (%) | – | ||
| Amlodipine | 19 (70.4) | 16 (55.2) | |
| Nifedipine | 4 (14.8) | 1 (3.4) | |
| Labetalol | 23 (85.2) | 15 (51.7) | |
| Metoprolol | 9 (33.3) | 15 (51.7) | |
| Carvedilol | 5 (18.5) | 1 (3.4) | |
| Angiotensin-converting enzyme inhibitor | 5 (18.5) | 2 (6.9) | |
IV, intravenous; SD, standard deviation.
Outcomes
Our primary outcome, ICU length of stay, was significantly shorter in the rapid transition compared to the slow transition group (3.6 vs. 7.7 days, P<0.001), accounting for a 4.1-day between-group difference, as seen in Table 3. Hospital length of stay was also shorter in the rapid group (8.5 vs. 16.5 days, P=0.11). Patients in the rapid transition group required a shorter median duration of IV vasoactive use (36.0 vs. 115.7 hours, P<0.001). Patients in the rapid transition group had a significantly lower vasoactive drug cost during ICU stay compared to the slow transition group ($562 vs. $2,449). The incidence of hypotension (SBP <90 mmHg) was not significantly different between the rapid transition group and the slow transition group (18.5% vs. 13.8%, P=0.77). There were no patients identified with a subsequent aortic event.
Table 3
| Outcome | Slow (n=27) | Rapid (n=29) | P |
|---|---|---|---|
| Intensive care unit length of stay (days), median [IQR] | 7.7 [5.4–11.5] | 3.6 [1.7–4.8] | <0.001 |
| Hospital length of stay (days), median [IQR] | 16.5 [8.5–22.7] | 8.5 [6.5–17.4] | 0.11 |
| IV vasoactive duration (hours), median [IQR] | 115.7 [92–141] | 36.0 [22–58] | <0.001 |
| Total IV drug cost ($), median [IQR] | 2,449 [1,597–3,378] | 562 [338–823] | – |
| Hypotension, n (%) | 5 (18.5%) | 4 (13.8%) | 0.77 |
| Hypotension requiring changes in enteral regimen, n (%) | 1 (3.7%) | 1 (3.4%) |
IQR, interquartile range; IV, intravenous.
Practice patterns
Data on the timeline of events and vasoactive practice patterns are depicted in Tables 4 and Table 5, respectively. Following hospital admission or establishment of enteral access post-surgical repair, PO vasoactive medications were initiated significantly faster in the rapid transition group compared to the slow transition group (median 9.0 vs. 19.0 hours, P=0.09). Both slow and rapid groups required a median of two vasoactive medication infusions to achieve hemodynamic goals (P=0.062). There were more PO medication adjustments in the rapid transition group compared to the slow transition group (median 1.3 vs. 0.72, P=0.04). For patients who were on stable rates of IV infusions for at least 24 hours, a greater proportion of patients in the rapid group experienced changes to PO medication regimen, compared to slow group (63.0% vs. 27.6%, P=0.008).
Table 4
| Events | Slow (n=27) | Rapid (n=29) | P |
|---|---|---|---|
| Delay from admission to first enteral antihypertensive medication administration (hours), median [IQR] | 42.3 [15.9–93.8] | 35.2 [14.2–80.5] | 0.65 |
| Delay from enteral access to first enteral antihypertensive medication administration (hours), median [IQR] | 19.0 [7.7–45.8] | 9.0 [1.9–15.0] | 0.09 |
| Delay from operating room exit to first enteral antihypertensive medication administration (hours), median [IQR] | 60.7 [40.4–154.5] | 32.1 [19.7–41.4] | 0.02 |
| Delay from end of IV infusion to intensive care unit discharge (hours), median [IQR] | 60.2 [16.5–144.1] | 35.2 [24.7–73.1] | 0.31 |
IQR, interquartile range; IV, intravenous.
Table 5
| Practice patterns | Slow (n=27) | Rapid (n=29) | P |
|---|---|---|---|
| Number of IV agents used, median [IQR] | 2 [2–2] | 2 [1–2] | 0.06 |
| Delay to administration of first oral agent (hours), median [IQR] | 19 [7.7–45.8] | 9 [1.9–15.0] | 0.09 |
| Number of oral medication adjustments in first 24 hours, mean ± SD | 0.72±1.1 | 1.3±1.3 | 0.04 |
| Number of PO medication adjustments in first 72 hours, mean ± SD | 2.63±2.3 | 2.8±2.1 | 0.76 |
| Resumed similar prior to admission medication regimen by 72 hours after initiation of IV infusion†, n (%) | 11 (40.7) | 14 (48.3) | 0.90 |
| Stable IV rates >24 hours without oral medication adjustment‡, n (%) | 17 (63.0) | 8 (27.6) | 0.008 |
†, ≥50% of similar classes at ≥50% prior to admission dose; ‡, stable infusion rate ±15% of 24-hour average dose. IV, intravenous; IQR, interquartile range; SD, standard deviation.
Discussion
In this study of patients with acute aortic dissection, rapid transition to PO vasoactive agents within 72 hours of IV infusion initiation may have been associated with a shorter median ICU length of stay. Patients in the rapid group had overall shorter durations of IV antihypertensive medication use, resulting in a median drug acquisition cost savings of $1,887 between cohorts. Considering the financial impact of a shorter ICU stay, the magnitude of cost savings differences between the rapid and slow group is even greater.
This study adds to the evidence suggesting rapid transition from IV to PO antihypertensive agents is associated with a shorter ICU length of stay. A previous study by Michaud et al., patients with aortic dissections demonstrated that rapid transition from IV to PO vasoactive agents resulted in a significantly shorter ICU length of stay and established the 72-hour definitions for rapid vs. slow transition (5). The authors found that in patients with aortic dissections, ICU length of stay was significantly shorter when patients were able to be transitioned from IV to PO agents within 72 hours (median stay 3.6 vs. 10.5 days, P<0.001). This study also reported that hospital length of stay was significantly shorter in the rapid group. The data from our study, which was completed in a different healthcare system, prove consistency of these results. Studies evaluating patients with intracerebral hemorrhage also describe the issue of prolonged need for IV vasoactive infusions and the potential benefit of enteral agents to facilitate ICU discharge. Zhu et al. found that early initiation of oral antihypertensives within 24 hours using a standard protocol in patients with hypertensive intracerebral hemorrhage was associated with a significantly shorter ICU stay and cost of hospitalization (4).
During our study timeframe, there was no institutional workflow or protocol in place to guide transition from IV to PO antihypertensives, and these units operate under an “open” ICU model. One of the challenges of initiating enteral agents is the lack of established equivalent conversions of common vasoactive infusion rates and oral antihypertensive agent doses to facilitate the transition (6). In this study, clinical decisions were largely at the discretion of the primary surgery team with regard to selection of specific IV or PO agents and hemodynamic goals. For this study, we chose the 72 hours as the threshold to delineate the rapid group and slow groups, as this transition point has been established in a similar study (5). However, the time of initiation of IV infusions was considered ‘time zero’ to better account for any delays in initiation of oral antihypertensive agents, particularly in this population, which may not have enteral access perioperatively.
Comparing vasoactive management between the rapid and slow groups, we saw several trends that impacted how quickly patients were able to be liberated from IV vasoactive agents. In the first 24 hours, the rapid group had more adjustments to their enteral antihypertensive regimen, including adding a second medication or intensification of the dose or frequency of the current regimen. By contrast, patients in the slow group were more frequently continued on the same regimen of enteral agents, despite stable vasoactive infusion requirements. The reason for the delay in escalating enteral hypertensive agents for each patient was not described in the progress notes, but likely case-specific. These missed opportunities to escalate enteral antihypertensive agents may have prolonged the need for IV vasoactive infusion in these patients. Of the oral antihypertensive medication options, amlodipine was frequently used, and the slow group had a proportionately higher rate of amlodipine use than the rapid group. Amlodipine has a slow onset of action, with significant reductions in BP only after 24 hours following the first dose, hence the once daily dosing (7). Medications that are administered on a once-daily frequency provide fewer opportunities in a 24-hour timeframe to assess for efficacy and uptitrate medications if hemodynamic goals are not achieved. In developing a protocol to facilitate rapid titration off IV infusions to an enteral regimen, use of more fast-acting, short-duration medication options (e.g., captopril or labetalol) may allow for more timely adjustments to the oral medication regimen to help wean vasoactive infusions. While the proportion of patients in the rapid and slow groups that underwent surgery was comparable, there was a greater percentage of patients that had surgical repair in the rapid group. Conservative management of aortic dissection may contribute to an increased length of stay in the slow group due to the cautious initiation of enteral medications to avoid hypertensive spikes.
One possible safety concern with early initiation and aggressive up titration of oral antihypertensives while on IV continuous vasoactive infusions is hypotension. However, the rate of hypotension was low in both cohorts in this study and did not differ between groups. Only a single patient in each group required dose reduction in their oral medication regimen following a hypotensive episode.
This study had several limitations. First, this was a retrospective study conducted via chart review. Any missing or incorrect documentation in the medical record would impact our data and findings. Second, severity of illness, surgical procedures and complications, and patient comorbidities were not assessed, and patients may have had other indications for an extended ICU stay that were not evaluated. Third, since there was no institutional workflow to guide a patient’s clinical course, practice varied. The differences in practice between the two main primary teams, vascular surgery and cardiothoracic surgery, were not assessed. The efficacy of BP and HR management was not assessed due to lack of standardized hemodynamic goals across physician groups. Additionally, individual patients may have had changing hemodynamic goals depending on clinical status. As such, efficacy of hemodynamic goal achievement between groups was not compared. However, it is unlikely that transitioning off IV vasoactive agents would not be considered in patients who were uncontrolled. The results of this study should be interpreted with caution. This study may not include a sufficient patient population to draw a meaningful conclusion for the selected outcome since the sample was smaller than required to detect a large effect size. With this study’s small sample size, all of the parameters calculated from this patient population may differ considerably from the general population of interest. With the paucity of literature evaluating this study question, the estimated effect size of interest used to calculate the needed sample size may be incorrect. And further, with the limited patient population, it is possible the effect size is overestimated.
Conclusions
Urgent hemodynamic management is a hallmark of guideline-directed therapy in patients with acute aortic dissection. While the use of IV agents allows rapid achievement of guideline-recommended BP and HR goals, patients started on continuous infusions require ICU admission for close monitoring. In patients achieving goal BP and HR on stable rates of continuous infusions, initiation and uptitration of PO antihypertensive medications may facilitate weaning of IV vasoactive medications. Earlier discontinuation of IV vasoactive continuous infusions would allow patients to be discharged from the ICU, reducing medication costs and hospitalization costs. The availability of PO antihypertensive agents can assist with achievement of hemodynamic control and liberation from IV continuous infusions would allow patients to discharge from the ICU, but there are limited data available to assist with how and when to safely transition from IV to PO agents. This study supports that patients with acute aortic dissection can be transitioned from vasoactive infusions to PO antihypertensive agents within 72 hours. Further, converting to an enteral regimen within this timeframe may be associated with a shorter ICU length of stay without an increase in hypotension.
Further studies are needed to confirm or refute these findings and to investigate if a specific regimen or titration strategy of oral antihypertensive medications may best facilitate timely achievement of hemodynamic goals to progress patients toward ICU discharge. Additionally, the delay to initiation of PO medication was a potential modifiable risk factor. The effect of early initiation of PO antihypertensive medications may be a pertinent research question in the aortic dissection patient population. Finally, additional retrospective data to confirm that early transition to enteral antihypertensives does not confer any harm to patients would be valuable. Such studies may consider evaluating the incidence of hypotension, hypertension and tachycardia above goal hemodynamic parameters, and incidence of subsequent aortic events.
Acknowledgments
The authors thank Paul Takamoto, PharmD, BCCCP and A. Kendall Gross, PharmD, BCCCP, BCPS for technical assistance with this project.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1274/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1274/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1274/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1274/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of University of California, San Francisco, Laurel Heights Committee (registration No. 00003471) and individual consent for this retrospective analysis was waived (IRB 20-31817, Reference #291091).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. Erratum in: Circulation 2010 Jul 27;122(4):e410. [PubMed]
- Suzuki T, Isselbacher EM, Nienaber CA, et al. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol 2012;109:122-7. [Crossref] [PubMed]
- Shah NH, Do LV, Petrovich J, et al. Reducing Cost and Intravenous Duration of Nicardipine in Intracerebral Hemorrhage Patients via an Interdisciplinary Approach. J Stroke Cerebrovasc Dis 2016;25:2290-4. [Crossref] [PubMed]
- Zhu Z, Bower M, Stern-Nezer S, et al. Early Initiation of Oral Antihypertensives Reduces Intensive Care Unit Stay and Hospital Cost for Patients with Hypertensive Intracerebral Hemorrhage. Neurocrit Care 2020;32:707-14. [Crossref] [PubMed]
- Michaud CJ, Packard AE, Timek T. Faster Transition From Intravenous to Oral Antihypertensives Associated With Improved Outcomes After Aortic Dissection. Ann Pharmacother 2020;54:22-8. [Crossref] [PubMed]
- Dasta JF, Boucher BA, Brophy GM, et al. Intravenous to oral conversion of antihypertensives: a toolkit for guideline development. Ann Pharmacother 2010;44:1430-47. [Crossref] [PubMed]
- Amlodipine. In: Lexi-Drugs. Hudson, Ohio: Wolters Klewer UpToDate, Inc.; Available online: https://online.lexi.com (accessed January 25, 2022).


