
Predictors associated with mortality of veno-venous extracorporeal membrane oxygenation therapy
Highlight box
Key findings
• Independent predictors associated with in-hospital mortality of patients treated with veno-venous extracorporeal membrane oxygenation (V-V ECMO) were age, newly developed liver failure, red blood cell transfusion and platelet concentrate transfusion. In-hospital mortality was 37.6% and did not vary significantly between different indications.
What is known and what is new?
• Patients undergoing V-V ECMO therapy still face high mortality. Factors such as older age, body mass index (BMI), and longer duration of invasive mechanical ventilation are well known hazards.
• We identified novel risk factors such as newly developed liver failure and transfusion of red blood cells and platelet concentrates to be associated with increased mortality.
What is the implication, and what should change now?
• Knowledge of independent mortality predictors helps to use V-V ECMO in a targeted and efficient manner.
Introduction
Veno-venous extracorporeal membrane oxygenation (V-V ECMO) constitutes a resource-intense therapy to support patients with failing lungs unresponsive to conventional therapy (1,2). Increasing use of V-V ECMO is based on growing evidence of its effectiveness to support patients with acute respiratory distress syndrome (ARDS) (3) and to bridge patients to lung transplantation (4-6). However, V-V ECMO use in adult patients with respiratory failure remains associated with significant mortality rates as well as a wide knowledge gap (7-9). The systematic review and meta-analysis of Tran et al. summarizes the association between prognostic factors and the risk of mortality in patients requiring V-V ECMO for coronavirus disease 2019 (COVID-19) (10). Patient factors such as older age, male sex, and chronic lung disease, and pre-cannulation disease factors, such as longer duration of symptoms, and longer duration of invasive mechanical ventilation are reported. In this regard, in-depth knowledge of predictive factors associated with mortality of V-V ECMO is crucial. Reputed scores as RESP (11), PRESET (12), or PRESERVE (13) assume specified patient parameters. In a recent retrospective study, we have identified so far unknown independent mortality predictors of extracorporeal life support therapy for the failing heart (14). Comprehensive analysis of mortality predictors could provide guidance in making informed decisions about ECMO indications, its management strategy and reinforce personalized medical care.
In this study, we focus on the outcome of V-V ECMO therapy in patients with respiratory failure over a period of 13 years at the University Hospital Zurich in Switzerland. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1273/rc).
Methods
Study design
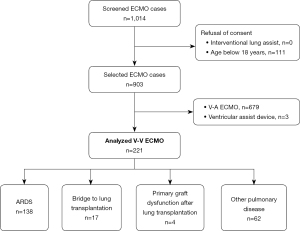
This retrospective, observational single-center study enrolled adult patients treated with V-V ECMO at the University Hospital Zurich in Switzerland between January 2007 and December 2019. The inclusion period was intentionally concluded prior to the outbreak of the SARS-CoV-2 2019 pandemic since V-V ECMO for SARS-CoV-2 pneumonia would represent a different patient cohort. Our hospital is a tertiary care referral institution that is a designated ECMO center. Data of all patients with V-V ECMO was collected from the clinic-specific registers and the hospital-wide clinical information system through medical controlling. Patients were excluded if they were under 18 years of age or rejected informed consent (Figure 1). Based on current literature, we defined four categories for ECMO use: ARDS, bridge to lung transplantation, primary graft dysfunction after lung transplantation, and other pulmonary disease indications (1,2). The latter category comprises exacerbation of pulmonary disease and subsequent gas exchange failure without meeting ARDS definitions. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed by the Cantonal Ethics Commission of Zurich, Switzerland (BASEC-Nr. 2019-01926). The requirement for written informed consent was waived due to the retrospective nature of the study.
Study endpoints
The objectives of this study were to report in-hospital mortality and 30-day mortality after ECMO implantation as well as to identify new independent predictive factors associated with death.
Data collection and variables
Medical history of patients was reviewed manually. Operative and intensive care unit reports allowed identifying pre-defined variables, which are specified in the supplementary material (Table S1). Coding data and administrative data were saved and exported from SAP ERP (SAP AG, Walldorf, Germany). Thirty-day survival was defined as 30-day survival after ECMO implantation.
ECMO indication
V-V ECMO was used in cases of unmanageable respiratory failure with maxed out conventional therapeutic procedures. Indications included “bridge to transplant” after full clinical assessment and listing for lung transplantation in the context of a sudden acute on chronic worsening. Exceptional cases without full clinical assessment were included only in case of e.g., an initial diagnosis of a refractory interstitial lung disease and absence of contraindications. Further indications comprised “bridge to recovery”, including primary graft dysfunction after lung transplantation, respiratory failure such as ARDS and other pulmonary disease indications. ARDS was quantified using the severity PaO2/FiO2 (PF) ratio or Horowitz ratio <50 for 3 h, PF ratio <80 for >6 h, acidosis.
Before ECMO insertion the severity of respiratory failure was assessed as follows: ventilation was optimized [tidal volume (Vt) <6 mL/kg, plateau pressure (Pplat) <28–30 cmH2O, positive end expiratory pressure PEEP >10 cmH2O, relaxation in the first 48 hours, early Prone position], despite optimized ventilation occurrence of refractory hypoxemia (FiO2 >80% and PaO2/FiO2 ratio <80 mmHg over 6 h), Horowitz index (<100 mmHg transfer to an ECMO center, <50–80 mmHg indication for ECMO insertion).
ECMO insertion
The standard approach consisted in the insertion of the drainage cannula percutaneously through the right common femoral vein and insertion of the supply cannula percutaneously through the right internal jugular vein. The tip of the femoral cannula was placed in the inferior vena cava just underneath the entrance into the right atrium. The tip of the jugular cannula was positioned in the superior vena cava at the junction with the right atrium. This method prevented recirculation through the ECMO system. If the right jugular vein could not be used, the left common femoral vein was used for percutaneous placement of the supply cannula. In this femoro-femoral configuration, the tip of the supply cannula was advanced into the right atrium just underneath the entrance of the superior vena cava, and the tip of the drainage cannula in the proximal inferior vena cava was placed a few centimeters below the confluence with the right atrium to avoid recirculation through the ECMO system. The left jugular vein was not used since advancement of the cannula through the curvature at the confluence of the left jugular vein into the left brachiocephalic vein might provoke perforation. In patients in whom mobilization was intended while being on ECMO, a single dual-lumen cannula consisting of one lumen for drainage and one lumen for supply was preferred. The cannula was placed percutaneously in the right internal jugular vein. ECMO placement was accomplished by cardiac surgeons. Cannulae were placed guided by transesophageal echocardiography or fluoroscopy.
Statistical analysis
Continuous variables were summarized as median with interquartile range (IQR) or mean with standard deviation (SD), and compared using the Mann-Whitney U test. Categorical variables were presented as numbers with percentages, and compared using the Fisher’s exact test. We performed a multivariable logistic regression analysis to determine the effect of possibly influential variables on the binary variable in-hospital mortality. There was no evidence for presence of multicollinearity as assessed by Spearman’s correlation. To detect potential changes over time, we incorporated a cubic spline for the time variable in the model. The associated effect of time was displayed in a graph. To evaluate discrimination of our regression model we used the receiver operating characteristic (ROC) curve analysis (Figure S1). Calibration was assessed using the Hosmer-Lemeshow test. Duration of ECMO for each category as well as the in-hospital mortality were estimated using the Kaplan-Meier method. Death was defined as a censoring event that terminated ECMO therapy. Hypothesis tests were 2-sided. Significance was set at a P value <0.05. Statistical analyses were performed with R version 4.0.5.
Results
During the study period of 13 years, 221 patients received V-V ECMO therapy. The patients’ median age was 50 years, and 38.9% were female. The main characteristics for all four indications “ARDS”, “bridge to lung transplantation”, “primary graft dysfunction after lung transplantation”, and “other pulmonary disease indications” are reported in Table 1.
Table 1
| Variables | All indications (n=221) | ARDS (n=138) | Bridge to lung transplantation (n=17) | Primary graft dysfunction after lung transplantation (n=4) | Other pulmonary disease indications (n=62) |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age (years) | 50.0 (38.0 to 60.0) | 50.0 (38.0 to 58.0) | 44.0 (37.0 to 50.0) | 49.0 (38.8 to 54.5) | 55.0 (38.5 to 64.8) |
| BMI (kg/m²) | 25.6 (22.2 to 29.4) | 26.5 (23.4 to 30.9) | 20.2 (18.6 to 23.0) | 20.7 (17.2 to 25.2) | 25.3 (21.8 to 28.7) |
| Sex (female), n (%) | 86 (38.9) | 59 (42.8) | 6 (35.3) | 4 (100.0) | 17 (27.4) |
| SAPS II (points) (n=212) | 48.0 (33.0 to 63.0) | 53.0 (39.0 to 67.0) | 26.5 (19.0 to 38.0) | 34.0 (25.0 to 41.5) | 42.0 (29.0 to 59.0) |
| PRESERVE (points) (n=220) | 3.0 (1.0 to 4.0) | 2.0 (1.0 to 4.0) | 3.0 (3.0 to 4.0) | 3.5 (1.8 to 5.2) | 4.0 (1.0 to 4.0) |
| Baseline laboratory parameters | |||||
| Lactate (mmol/L) (n=177) | 1.6 (1.0 to 3.2) | 1.9 (1.2 to 3.8) | 0.9 (0.8 to 1.2) | 1.0 (0.7 to 1.2) | 1.7 (1.0 to 3.1) |
| Hemoglobin (g/L) (n=205) | 92.0 (81.0 to 107.0) | 89.0 (79.0 to 106.6) | 101.0 (88.0 to 107.0) | 82.0 (80.5 to 87.8) | 94.0 (85.8 to 110.0) |
| Myoglobin (µg/L) (n=189) | 196.0 (70.0 to 725.0) | 241.0 (100.5 to 786.0) | 39.0 (21.0 to 60.8) | 131.0 (81.0 to 181.5) | 161.5 (70.0 to 808.8) |
| Creatinine (µmol/L) (n=218) | 92.5 (60.0 to 161.5) | 106.0 (65.0 to 172.0) | 50.0 (35.0 to 78.0) | 75.0 (69.0 to 93.0) | 87.0 (60.0 to 145.5) |
| Comorbidities, n (%) | |||||
| Coronary artery disease | 17 (7.7) | 10 (7.2) | 0 (0.0) | 1 (25.0) | 6 (9.7) |
| Congestive heart failure | 15 (6.8) | 8 (5.8) | 0 (0.0) | 0 (0.0) | 7 (11.3) |
| Obstructive pulmonary disease | 20 (9.0) | 7 (5.1) | 3 (17.6) | 0 (0.0) | 10 (16.1) |
| Diabetes mellitus | 29 (13.1) | 14 (10.1) | 2 (11.8) | 1 (25.0) | 12 (19.4) |
| Chronic kidney disease | 18 (8.1) | 10 (7.2) | 0 (0.0) | 0 (0.0) | 4 (6.5) |
Data presents as median (IQR) or n (%). V-V ECMO, veno-venous extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; BMI, body mass index; SAPS II, simplified acute physiology score II; PRESERVE, predicting death for severe ARDS on VV-ECMO score; IQR, interquartile range.
Overall, in-hospital mortality of patients receiving V-V ECMO therapy was 37.6% (83 out of 221 patients). In-hospital mortality varied between indications for V-V ECMO as follows: 25.0% (1/4) for primary graft dysfunction after lung transplantation, 29.4% (5/17) for bridge to lung transplantation, 36.2% (50/138) for ARDS and 43.5% (27/62) for other pulmonary disease indications (P=0.61); 30.8% (68 out of 221) of patients died during V-V ECMO therapy, primarily patients with ARDS and other pulmonary disease indications. In-hospital mortality after successful V-V ECMO weaning was 6.8% (15 out of 221) (Table 2). Causes of death among those who died after ECMO weaning were not consistent and often not clearly attributable to a specific pathology. Multiorgan failure aggravated through disseminated coagulopathy and hemorrhagic or septic shock resulted in an unfavorable outcome.
Table 2
| Variables | All indications (n=221) | ARDS (n=138) | Bridge to lung transplantation (n=17) | Primary graft dysfunction after lung transplantation (n=4) | Other pulmonary disease indications (n=62) |
|---|---|---|---|---|---|
| ECMO, n (%) | |||||
| External insertion† | 54 (24.4) | 43 (31.2) | 0 (0.0) | 0 (0.0) | 11 (17.7) |
| Insertion technique | |||||
| Seldinger peripheral | 218 (98.6) | 138 (100.0) | 15 (88.2) | 3 (75.0) | 62 (100.0) |
| Surgical peripheral | 3 (1.4) | 0 (0.0) | 2 (11.8) | 1 (25.0) | 0 (0.0) |
| Surgical central | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Successful weaning | 136 (61.5) | 96 (69.6) | 2 (11.8) | 3 (75.0) | 35 (56.5) |
| Mortality, n (%) | |||||
| In-hospital mortality | 83 (37.6) | 50 (36.2) | 5 (29.4) | 1 (25.0) | 27 (43.5) |
| Death during ECMO therapy | 68 (30.8) | 42 (30.4) | 1 (5.9) | 1 (25.0) | 24 (38.7) |
| In-hospital death after ECMO weaning | 15 (6.8) | 8 (5.8) | 4 (23.5) | 0 (0.0) | 3 (4.8) |
| 30-day mortality (n=210)†† | 66 (31.4) | 45 (29.5) | 4 (23.5) | 1 (25.0) | 27 (38.6) |
| Duration | |||||
| ECMO (days) | 7.0 (3.0 to 15.0) | 7.0 (4.0 to 14.8) | 23.0 (12.0 to 31.0) | 12.5 (11.5 to 13.0) | 5.0 (2.0 to 11.5) |
| Ventilation (days) | 13.0 (6.0 to 28.0) | 14.5 (7.2 to 30.8) | 20.0 (5.0 to 28.0) | 42.0 (24.8 to 54.5) | 9.5 (3.2 to 19.0) |
| ICU (days) | 17.0 (9.0 to 35.0) | 17.0 (10.0 to 33.8) | 32.0 (21.0 to 56.0) | 47.0 (36.0 to 55.0) | 13.0 (7.0 to 30.2) |
| Hospital stay (days) | 26.0 (13.0 to 48.0) | 27.5 (13.0 to 48.0) | 38.8 (32.0 to 73.0) | 62.5 (44.8 to 80.2) | 20.0 (11.0 to 35.0) |
Data presents as median (IQR) or n (%). †, ECMO insertion by University Hospital Zurich outreach team in another hospital before transfer to University Hospital Zurich for definitive care; ††, deviating number of patients (210 instead of the total number of 221) due to loss of follow up on day 30 after ECMO insertion. V-V ECMO, veno-venous extracorporeal membrane oxygenation; ECMO, extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IQR, interquartile range.
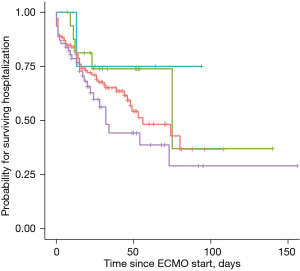
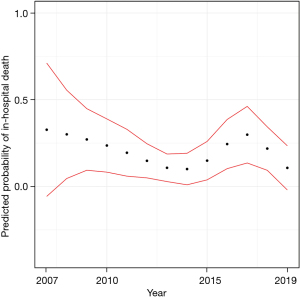
The Kaplan-Meier estimates of in-hospital mortality by ECMO indication are depicted in Figure 2. There was no significant difference between subgroups. Furthermore, we analyzed the predicted in-hospital mortality stratified by the year of ECMO insertion (Figure 3). In a logistic regression model with the cubic spline interpolation, we found no evidence for a trend over time.
The Simplified Acute Physiology Score (SAPS) II score was significantly different between the subgroups (P<0.001), without a relevant variation over time. The number of V-V ECMOs per year and the course of the SAPS II score over time is shown in Figure 4. Major bleeding events occurred with the highest percentage of 29.4% (5 out 17 patients) in the bridge to lung transplantation group, followed by the group of patients with other pulmonary disease indications with 22.6% (14 out of 62), the ARDS group with 13% (18 out of 138) and none major bleeding events in patients with primary graft dysfunction after lung transplantation (Table 3). Median duration of V-V ECMO therapy was 7 days (IQR, 3.0–15.0 days). Patients receiving ECMO as a bridge to lung transplantation required the longest treatment with 23 days (IQR, 12.0–31.0 days), whereas the group with other pulmonary disease indications represented the shortest duration of ECMO support with 5 days (IQR, 2.0–11.5 days). Median length of lung ventilation was 13 days (6.0–28.0 days), varying between the maximum length of 42 days (24.8–54.5 days) in patients with primary graft dysfunction after lung transplantation and the minimum length of 9.5 days (IQR, 3.2–19.0 days) in the group with other pulmonary disease indications. The majority of 61.5% (136 out of 221) of patients was successfully weaned from ECMO. On average, patients spent 26 days (IQR, 13.0–48.0 days) in hospital. The subgroups “bridge to lung transplantation” and “primary graft dysfunction after lung transplantation” exhibited the longest hospital stays [38.8 days (IQR, 32.0–73.0 days) and 62.5 days (IQR, 44.8–80.2 days) respectively].
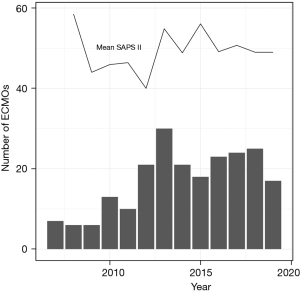
Table 3
| Complications | All indications (n=221) | ARDS (n=138) | Bridge to lung transplantation (n=17) | Primary graft dysfunction after lung transplantation (n=4) | Other pulmonary disease indications (n=62) |
|---|---|---|---|---|---|
| Transfusions | |||||
| Red blood cells (units) | 3.0 (1.0 to 8.0) | 3.0 (1.0 to 7.0) | 8.0 (3.0 to 10.0) | 9.5 (6.5 to 11.2) | 3.0 (1.0 to 5.8) |
| 5.3±5.3 | 5.3±5.3 | 7.8±5.0 | 8.2±4.5 | 4.7±5.4 | |
| Fresh frozen plasma (units) | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 0.0) | 1.0 (0.0 to 1.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 1.0) |
| 0.5±1.2 | 0.4±1.2 | 0.9±1.1 | 0.0 | 0.7±1.3 | |
| Platelet concentrate (units) | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 1.0) |
| 1.4±3.4 | 1.6±3.6 | 0.7±1.0 | 0.0 | 1.3±3.6 | |
| Major bleeding, n (%) | 37 (16.7) | 18 (13.0) | 5 (29.4) | 0 (0.0) | 14 (22.6) |
| Intra-cranial bleeding, n (%) | 8 (3.6) | 8 (5.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stroke, n (%) | 5 (2.3) | 3 (2.2) | 0 (0.0) | 0 (0.0) | 2 (3.2) |
| Newly developed liver failure, n (%) | 24 (10.9) | 16 (11.6) | 1 (5.9) | 0 (0.0) | 7 (11.3) |
| Renal replacement therapy, n (%) | 95 (43.0) | 64 (46.4) | 6 (35.3) | 2 (50.0) | 23 (37.1) |
| Ischemia, n (%) | |||||
| Extremities | 14 (6.3) | 9 (6.5) | 0 (0.0) | 0 (0.0) | 5 (8.1) |
| Intestinal | 10 (4.5) | 7 (5.1) | 0 (0.0) | 0 (0.0) | 3 (4.8) |
Data presents as median (IQR), mean ± SD, or n (%). V-V ECMO, veno-venous extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; IQR, interquartile range; SD, standard deviation.
Assessing the effect of possibly influential variables on in-hospital mortality, we chose predictor variables as parameters of interest for clinical reasons. It is worth mentioning that our main interest was not performing a classical prediction model but rather to focus on specific preselected parameters and their effect on in-hospital death. Their choice is based on clinical relevance, regular and reliable measurement processes in our hospital. The model shows good discrimination with an area under the curve (AUC) belonging to ROC curve which is 0.85 with a 95% confidence interval from 0.8 to 0.91. The Hosmer-Lemeshow test indicates a good model fit (P=0.66). Consequently, a multiple logistic regression model with patients’ clinical characteristics showed that age with an odds ratio (OR) of 1.05 [95% confidence interval (CI): 1.02–1.07; P=0.001] as well as newly detected liver failure with an OR of 4.83 (95% CI: 1.27–20.3; P=0.02) were associated with significantly higher mortality (Table 4). Moreover, our analysis revealed that each unit of red blood cell as well as platelet concentrate transfusion given per day were highly significant mortality predictors: red blood cell transfusion with an OR of 1.91 (95% CI: 1.39–2.74; P<0.001) per 0.1 units per day and platelet concentrate transfusion with an OR of 1.93 (95% CI: 1.28–3.15; P=0.004) per 0.1 unit per day. Compared to ARDS, representing the reference category, all three other indications were not a predictor for mortality. Adjusting the regression model by including the SAPS II does not show any relevant change in significance (Table S2).
Table 4
| Predictor variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age (per year) | 1.05 | 1.02 to 1.07 | 0.001 |
| Indication | |||
| ARDS | Reference | ||
| Bridge to lung transplantation | 1.56 | 0.39 to 5.66 | 0.51 |
| Primary graft dysfunction after LTX | 1.58 | 0.07 to 14.34 | 0.71 |
| Other pulmonary disease | 1.46 | 0.61 to 3.46 | 0.39 |
| Transfusions | |||
| Red blood cells (0.1 unit/day) | 1.91 | 1.39 to 2.74 | <0.001 |
| Fresh frozen plasma (0.1 unit/day) | 1.26 | 0.62 to 3.55 | 0.65 |
| Platelet concentrate (0.1 unit/day) | 1.93 | 1.28 to 3.15 | 0.004 |
| Newly developed liver failure | 4.83 | 1.27 to 20.3 | 0.02 |
V-V ECMO, veno-venous extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; LTX, lung transplantation; CI, confidence interval.
Discussion
In this retrospective study, we investigated 221 cases of adult patients with V-V ECMO support over a period of 13 years in a single tertiary care referring ECMO center. We observed an overall in-hospital mortality of 37.6%. The registry data of the Extracorporeal Life Support Organization (ELSO) international report described an in-hospital mortality of 41.0% for adult respiratory V-V ECMO support (15). Data from Germany analyzing 22,960 patient cases on V-V ECMO therapy reported a higher in-hospital mortality of 53.9% (8). The dataset included a subgroup of patients with ARDS (10,801 out of 22,960 patients) with an in-hospital mortality of 54.4%. This mortality is clearly higher than the mortality rate of 36.2% reported in this manuscript. The discrepancy in mortality rates may be attributed to the age distribution of patients as advanced age is a known key prognostic indicator associated with higher mortality (16-18). Compared to our cohort with an age ranging from 38.0 to 60.0 years, the German study featured 28.7% of patients aged between 65 and 95 years.
In-hospital mortality was not significantly different between the reported indications for V-V ECMO therapy. It is worth mentioning that there is no complete agreement on predefined indication groups. However, we chose the three subgroups “ARDS”, “bridge to lung transplantation” and “primary graft dysfunction after lung transplantation” based on current literature (1,2,19-22) and in accordance with the current guidelines from the ELSO (18). We are aware that the number of patients in the group “primary graft dysfunction after lung transplantation” is low (4 out of 221 patients). Nonetheless, we consider this indication as an independent group as important. Primary graft dysfunction continues to be the most common indication for ECMO use after lung transplant procedures (20). Compared to the other three indications, these pathologies may result in important differences in ECMO therapy and prognosis of these higher risk patients. Finally, we included the fourth group “other pulmonary disease” referring to various specific indications for V-V ECMO therapy that did not meet the inclusion criteria of the three main groups of our study cohort.
The mortality rates did not vary significantly during our observation period between 2007 and 2019. To determine a possible non-linear change, we used cubic spline interpolation in the logistic regression model. One may speculate that there is an overall slight decrease in mortality by the end of the study in 2019 compared to the start of the study in 2007, as shown in Figure 3. However, the broader confidence intervals in the years before 2010 produced by a smaller sample size do not enable precise estimates. Thus, detecting a possible difference in mortality is precluded. This is in contrast to our expectation that technological improvements and an advanced understanding of ECMO support would have exerted an effect of time on mortality.
The systematic review and meta-analysis of Mitra et al. showed that patients with renal replacement therapy on ECMO had a higher mortality and a longer intensive care unit (ICU)/hospital stay compared to those without (23).
One focus of the study was the assessment of independent mortality predictors in patients with V-V ECMO therapy. To accomplish this endeavor, we used the multiple logistic regression model (Table 4). Consistent with previously published data, we found that increasing age in patients undergoing V-V ECMO treatment was associated with higher mortality (18). A recent retrospective study showed that patients 65 years of age and older have low rates of survival to hospital discharge (24). As there is a trend towards older patients receiving V-V ECMO therapy, it is currently subject of debate whether criteria and scoring systems should include age as a variable. Beside age also BMI is a factor strongly influencing the decision about ECMO usage. Currently, numerous publications indicate no effect of high BMI on mortality associated with the use of ECMO (25). Our cohort showed a mean BMI of 25.6 kg/m² (22.2 to 29.4 kg/m²) indicating that our patient collective was not obese. Therefore, analyzing the impact of obesity on mortality seems not meaningful with our patient collective and we did not add BMI to the multiple logistic regression model.
We identified newly detected liver failure as an independent predictor associated with mortality. Few data are available on the prognostic role of liver failure pre-cannulation but also during V-V ECMO treatment (26-28). Lazzeri et al. (27) reported that persistence of increased markers of hepatic dysfunction in ARDS patients with V-V ECMO therapy results in a significantly higher risk of death. Dizier et al. (26) showed that hepatic dysfunction was independently associated with 90-day mortality in patients with ARDS. Beside V-V ECMO, we found a new manifestation of liver failure during veno-arterial (V-A) ECMO treatment to be significantly associated with mortality (14). This is in line with the findings of Roth et al. reporting alkaline phosphatase and total bilirubin as strong predictors for 30-day mortality as well as long term mortality in patients undergoing ECMO following cardiovascular surgery (29). Acquired liver injury represents the most common form of hepatic dysfunction in the intensive care unit (30). It can occur after a hypoxic (e.g., shock), toxic (e.g., hepatotoxic drugs) or inflammatory insult (e.g., sepsis). Bilirubin is a marker of liver dysfunction and established as prognostic factor [e.g., Sequential Organ Failure Assessment (SOFA), Model for End-Stage Liver Disease (MELD)] (31,32). Hepatocellular damage interferes with other organ functions through metabolic signaling functions, inflammatory properties and immune response (33). Specific pulmonary dysfunction related to liver disease involves diffusion abnormalities along with the development of hepatopulmonary syndrome and portopulmonary hypertension (34). However, it is still unclear to what extent ECMO therapy impairs the liver function. These results indicate that newly detected liver failure may essentially contribute to the failure of V-V ECMO which needs to be subject to further investigation.
The regression analysis revealed that the number of transfused red blood cells as well as platelet concentrates is significantly associated with increased mortality. A large body of literature suggests that transfusion of allogenic blood products per se is independently associated with adverse outcomes including increased morbidity and mortality (35-38). In the setting of ECMO support, Guimbretière et al. described a prognostic impact of blood product transfusion (39). A recent systematic review and meta-analysis demonstrated ongoing uncertainty about transfusion triggers in ECMO patients (40). We conclude that a restrictive transfusion threshold during ECMO leads to a favorable clinical outcome in this single-center cohort. Another observational study in adults with V-V ECMO support showed that higher red blood cell transfusions were associated with mortality (41). We recently provided evidence for each allogenic blood transfusion unit given per day being an independent mortality predictor in V-A ECMO patients (14). We expected that transfusion and bleeding complications during V-V ECMO therapy may be closely related to each other. Hemorrhage in V-V ECMO patients has been shown to be strongly associated with higher mortality (42,43). However, we found in our analysis that bleeding complication per se was not an independent mortality predictor. This implies that allogenic blood transfusions may have a direct impact on mortality. Moreover, the recent findings by Martucci et al. in the PROTECMO study provide further support to the harms of blood transfusion in adults specifically with V-V ECMO treatment (44). In this international, multicentre, prospective, cohort study done in 41 ECMO centers, 83% of patients received at least one packed red blood cell (PRBC) unit. A restrictive transfusion strategy (PRBC transfusion with haemoglobin concentration of less than 7 g/dL) was associated with reduced risk of death. These findings endorse our results highlighting the value of allogenic blood transfusion as an independent mortality predictor.
Our study contains some limitations. Its retrospective design describes potential associations but cannot be used to definitely prove causation. Moreover, the study was only conducted in a single center. Due to the sample size, we have a lack of power to detect small differences between survivors and non survivors. Advances in technology and increased knowledge of V-V ECMO use during a study period of 13 years may have a beneficial effect on mortality. However, our data could not show such a benefit over time. The indication for ECMO implantation has changed in the past, becoming more liberal over time. Further, we analyzed a heterogenous patient collective. Due to the sample size, we have a lack of power to detect small differences between survivors and non survivors.
Conclusions
Patients undergoing V-V ECMO therapy still face high mortality. Our results corroborated previous data describing age as an independent mortality predictor. We identified novel risk factors such as newly developed liver failure and transfusion of red blood cells and platelet concentrates that are significantly associated with increased mortality. Knowledge of such independent predictors of mortality may help to develop strategies such as Patient Blood Management to improve outcome of patients on V-V ECMO.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1273/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1273/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1273/coif). AK has received support from Bayer AG (Switzerland) for lecturing. DRS’ academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland, the Swiss Society of Anesthesiology and Perioperative Medicine (SSAPM), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland, Vifor SA, Villars-sur-Glâne, Switzerland and Vifor (International) AG, St. Gallen, Switzerland. DRS is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland, CSL Behring GmbH, Marburg, Germany, LFB Biomédicaments, Courtaboeuf Cedex, France and Octapharma AG, Lachen, Switzerland. DRS received honoraria/travel support for consulting or lecturing from: Alliance Rouge, Bern, Switzerland, Danube University of Krems, Austria, European Society of Anesthesiology and Intensive Care, Brussels, BE, International Foundation for Patient Blood Management, Basel, Switzerland, Korean Society of Anesthesiologists, Seoul, Korea, Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, Paris, France, Society for the Advancement of Blood Management, Mount Royal NJ, Alexion Pharmaceuticals Inc., Boston, MA, AstraZeneca AG, Baar, Switzerland, Bayer AG, Zürich, Switzerland, B. Braun Melsungen AG, Melsungen, Germany, Baxter AG, Glattpark, Switzerland, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, CSL Vifor (Switzerland) Villars-sur-Glâne, Switzerland, CSL Vifor (International), St. Gallen, Switzerland, Celgene International II Sàrl, Couvet, Switzerland, Daiichi Sankyo AG, Thalwil, Switzerland, Haemonetics, Braintree, MA, USA, LFB Biomédicaments, Courtaboeuf Cedex, France, Merck Sharp & Dohme, Kenilworth, New Jersey, USA, Novo Nordisk Health Care AG, Zurich, Switzerland, Octapharma AG, Lachen, Switzerland, Pharmacosmos A/S, Holbaek, Denmark, Pierre Fabre Pharma, Alschwil, Switzerland, Portola Schweiz GmbH, Aarau, Switzerland, Roche Diagnostics International Ltd, Reinach, Switzerland, Sarstedt AG & Co., Sevelen, Switzerland and Nümbrecht, Germany, Shire Switzerland GmbH, Zug, Switzerland, Takeda, Glattpark, Switzerland, Werfen, Bedford, MA, Zuellig Pharma Holdings, Singapore, Singapore. MJW received speaker’s honoraria and reimbursement for travel expenses from Berlin Heart GmbH, Berlin, Germany, not related to this article. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed by the Cantonal Ethics Commission of Zurich, Switzerland (BASEC-Nr. 2019-01926). The requirement for written informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brodie D, Slutsky AS, Combes A. Extracorporeal Life Support for Adults With Respiratory Failure and Related Indications: A Review. JAMA 2019;322:557-68. [Crossref] [PubMed]
- Quintel M, Bartlett RH, Grocott MPW, et al. Extracorporeal Membrane Oxygenation for Respiratory Failure. Anesthesiology 2020;132:1257-76. [Crossref] [PubMed]
- Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med 2019;7:163-72. [Crossref] [PubMed]
- Abrams D, Brodie D. Extracorporeal Membrane Oxygenation for Adult Respiratory Failure: 2017 Update. Chest 2017;152:639-49. [Crossref] [PubMed]
- Abrams D, Bacchetta M, Brodie D. When the momentum has gone: what will be the role of extracorporeal lung support in the future? Curr Opin Crit Care 2018;24:23-8. [Crossref] [PubMed]
- Abrams D, Ferguson ND, Brochard L, et al. ECMO for ARDS: from salvage to standard of care? Lancet Respir Med 2019;7:108-10. [Crossref] [PubMed]
- Aoyama H, Uchida K, Aoyama K, et al. Assessment of Therapeutic Interventions and Lung Protective Ventilation in Patients With Moderate to Severe Acute Respiratory Distress Syndrome: A Systematic Review and Network Meta-analysis. JAMA Netw Open 2019;2:e198116. [Crossref] [PubMed]
- Friedrichson B, Mutlak H, Zacharowski K, et al. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care 2021;25:38. [Crossref] [PubMed]
- Tramm R, Ilic D, Davies AR, et al. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev 2015;1:CD010381. [Crossref] [PubMed]
- Tran A, Fernando SM, Rochwerg B, et al. Prognostic factors associated with mortality among patients receiving venovenous extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Lancet Respir Med 2023;11:235-44. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Hilder M, Herbstreit F, Adamzik M, et al. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Crit Care 2017;21:301. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Sahli SD, Kaserer A, Braun J, et al. Predictors associated with mortality of extracorporeal life support therapy for acute heart failure: single-center experience with 679 patients. J Thorac Dis 2022;14:1960-71. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-7. [Crossref] [PubMed]
- Deatrick KB, Galvagno SM, Mazzeffi MA, et al. Pilot study evaluating a non-titrating, weight-based anticoagulation scheme for patients on veno-venous extracorporeal membrane oxygenation. Perfusion 2020;35:13-8. [Crossref] [PubMed]
- Fan E, Gattinoni L, Combes A, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: A clinical review from an international group of experts. Intensive Care Med 2016;42:712-24. [Crossref] [PubMed]
- Tonna JE, Abrams D, Brodie D, et al. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J 2021;67:601-10. [Crossref] [PubMed]
- Biscotti M, Sonett J, Bacchetta M. ECMO as bridge to lung transplant. Thorac Surg Clin 2015;25:17-25. [Crossref] [PubMed]
- Hartwig MG, Walczak R, Lin SS, et al. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg 2012;93:366-71. [Crossref] [PubMed]
- Lafarge M, Mordant P, Thabut G, et al. Experience of extracorporeal membrane oxygenation as a bridge to lung transplantation in France. J Heart Lung Transplant 2013;32:905-13. [Crossref] [PubMed]
- Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg 2013;145:1065-71. [Crossref] [PubMed]
- Mitra S, Ling RR, Tan CS, et al. Concurrent Use of Renal Replacement Therapy during Extracorporeal Membrane Oxygenation Support: A Systematic Review and Meta-Analysis. J Clin Med 2021;10:241. [Crossref] [PubMed]
- Deatrick KB, Mazzeffi MA, Galvagno SM Jr, et al. Outcomes of Venovenous Extracorporeal Membrane Oxygenation When Stratified by Age: How Old Is Too Old? ASAIO J 2020;66:946-51. [Crossref] [PubMed]
- Zaidi SAA, Saleem K. Obesity as a Risk Factor for Failure to Wean from ECMO: A Systematic Review and Meta-Analysis. Can Respir J 2021;2021:9967357. [Crossref] [PubMed]
- Dizier S, Forel JM, Ayzac L, et al. Early Hepatic Dysfunction Is Associated with a Worse Outcome in Patients Presenting with Acute Respiratory Distress Syndrome: A Post-Hoc Analysis of the ACURASYS and PROSEVA Studies. PLoS One 2015;10:e0144278. [Crossref] [PubMed]
- Lazzeri C, Bonizzoli M, Cianchi G, et al. Bilirubin in the early course of venovenous extracorporeal membrane oxygenation support for refractory ARDS. J Artif Organs 2018;21:61-7. [Crossref] [PubMed]
- Dobrilovic N, March R, Yin K, et al. Liver Dysfunction Associated With In-Hospital Mortality in Adult Extracorporeal Membrane Oxygenation Support. Crit Care Explor 2021;3:e0484. [Crossref] [PubMed]
- Roth C, Schrutka L, Binder C, et al. Liver function predicts survival in patients undergoing extracorporeal membrane oxygenation following cardiovascular surgery. Crit Care 2016;20:57. [Crossref] [PubMed]
- Perez Ruiz de Garibay A, Kortgen A, Leonhardt J, et al. Critical care hepatology: definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit Care 2022;26:289. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-70. [Crossref] [PubMed]
- Horvatits T, Drolz A, Trauner M, et al. Liver Injury and Failure in Critical Illness. Hepatology 2019;70:2204-15. [Crossref] [PubMed]
- Møller S, Bendtsen F. Cirrhotic Multiorgan Syndrome. Dig Dis Sci 2015;60:3209-25. [Crossref] [PubMed]
- Chaiwat O, Lang JD, Vavilala MS, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009;110:351-60. [Crossref] [PubMed]
- Charles A, Shaikh AA, Walters M, et al. Blood transfusion is an independent predictor of mortality after blunt trauma. Am Surg 2007;73:1-5. [Crossref] [PubMed]
- Johnson DJ, Scott AV, Barodka VM, et al. Morbidity and Mortality after High-dose Transfusion. Anesthesiology 2016;124:387-95. [Crossref] [PubMed]
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet 2013;381:1855-65. [Crossref] [PubMed]
- Guimbretière G, Anselmi A, Roisne A, et al. Prognostic impact of blood product transfusion in VA and VV ECMO. Perfusion 2019;34:246-53. [Crossref] [PubMed]
- Abbasciano RG, Yusuff H, Vlaar APJ, et al. Blood Transfusion Threshold in Patients Receiving Extracorporeal Membrane Oxygenation Support for Cardiac and Respiratory Failure-A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth 2021;35:1192-202. [Crossref] [PubMed]
- Martucci G, Panarello G, Occhipinti G, et al. Anticoagulation and Transfusions Management in Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: Assessment of Factors Associated With Transfusion Requirements and Mortality. J Intensive Care Med 2019;34:630-9. [Crossref] [PubMed]
- Kreyer S, Muders T, Theuerkauf N, et al. Hemorrhage under veno-venous extracorporeal membrane oxygenation in acute respiratory distress syndrome patients: a retrospective data analysis. J Thorac Dis 2017;9:5017-29. [Crossref] [PubMed]
- Nunez JI, Gosling AF, O'Gara B, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med 2022;48:213-24. [Crossref] [PubMed]
- Martucci G, Schmidt M, Agerstrand C, et al. Transfusion practice in patients receiving VV ECMO (PROTECMO): a prospective, multicentre, observational study. Lancet Respir Med 2023;11:245-55. [Crossref] [PubMed]


