
Comparison of clinical effects between regional arterial embolization combined with surgery and simple surgery in the treatment of tuberculosis-destroyed lung—a retrospective comparative cohort study
Highlight box
Key findings
• Regional arterial embolization preconditioning combined with surgery can benefit the treatment of tuberculosis-destroyed lung.
What is known and what is new?
• Surgical resection provides the possibility of cure for tuberculosis. The surgical treatment of tuberculosis-destroyed lung is difficult and involves more bleeding and a longer operation time.
• The regional arterial embolization is widely used in the treatment of patients with hemoptysis.
What is the implication, and what should change now?
• The combination of the two methods can safely and effectively improve the current situation of difficult surgical treatment of tuberculosis-destroyed lung, and it is treatment method worth promoting for this condition.
Introduction
Tuberculosis-destroyed lung refers to irreversible destructive and extensive changes in lung tissue caused by repeated infection of mycobacterium tuberculosis. It can manifest as single or multiple caseous cavities or tuberculous fibrous cavities, localized bronchiectasis, mediastinal displacement, thickening of pleural adhesions, and loss of pulmonary function (1-3). Conservative treatment with drugs is not effective (4). Some patients continue to shed bacteria, which becomes a persistent source of infection and can even induce the drug resistance of tuberculosis (5,6). Some patients with cavities are prone to secondary infection mold, with hemoptysis or even massive hemoptysis potentially endangering life (7).
In recent years, regional arterial embolization has been widely used for the interventional treatment of patients with intractable local bleeding (8), but its clinical effect on patients with tuberculosis-destroyed lung and hemoptysis is limited. Due to the dual blood supply of the lung, the local collateral circulation of the lesion is rich, and the recurrence rate of the disease is high after the simple vascular embolism treatment (9).
For patients with tuberculosis-destroyed lung without surgical contraindications, the removal of the diseased lung in the operation provides the possibility for the complete cure of the disease (10). However, due to the long course and large lesion area of the tuberculosis-destroyed lung, most cases of pleural cavity adhesion are severe, and even tuberculous empyema and pleural collapse of the affected side can occur (11). During the operation, dissection of the adhesion and thickening of the pleura, exposure of the surgical field of vision, and dissociation of the diseased lungs are particularly difficult, and the operation process is lengthy. Moreover, due to extensive collateral circulation, nutrient vessel cluster and large surgical wound, more bleeding occurs during the operation, and the difficulty in hemostasis during the operation can further prolong the operation time and increase the amount of intraoperative bleeding, thus greatly increasing the risk of operation and the incidence of postoperative complications (12).
Through a retrospective comparative study, we found that the combination of regional arterial embolism and operation in the surgical treatment of tuberculosis-destroyed lung can significantly shorten the operation time, reduce intraoperative bleeding, and decrease the incidence of postoperative complications. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-224/rc).
Methods
A retrospective comparative cohort study was used to collect 28 patients with tuberculosis-destroyed lung who were treated surgically in our department from June 2021 to September 2022, and the patients were divided into 2 groups according to whether regional systemic arterial embolization was introduced before surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Henan Provincial Chest Hospital (No. 20220919) and individual consent for this retrospective analysis was waived. In the observation group (n=13), 13 cases had hemoptysis at admission, with the hemoptysis volume in 24 h being 100–300 mL in 8 cases, 300–500 mL in 3 cases, and more than 500 mL in 2 cases. The maximum hemoptysis volume in 24 h was about 800 mL. All patients in the observation group were treated with systemic arterial embolization in the focus area through the catheter before surgery, and surgery was performed 1 to 7 days after embolization. In the control group (n=15), 5 cases had hemoptysis, with the hemoptysis volume in 24 h being less than 100 mL in 3 cases and 100–150 mL in 2 cases. All patients in the control group underwent direct surgery after complete examination. To assess the application value of regional systemic artery embolization combined with surgery in the treatment of tuberculosis-destroyed lung, outcomes of operation time, intraoperative blood loss, and postoperative complication rate between the 2 groups were evaluated.
All patients underwent chest enhanced multi-slice spiral computed tomography (MSCT) examination before operation to determine the location of the lesion and its relationship with the surrounding tissues, 25 patients underwent fiberoptic bronchoscopy to further clarify the pathological changes in the bronchial lumen, 3 cases had bronchoscopy abandoned before operation due to high risk of bronchoscopy, and 13 patients in the observation group were treated with regional systemic arterial embolization before operation. The process of regional systemic artery embolization is as follows, after local anesthesia at the puncture site, the femoral artery was punctured, and then a 5F arterial sheath tube was placed. After the load dose of bivalirudin was injected, the maintenance amount of bivalirudin was continuously pumped. After the guide wire was sent, a 5F Cook angiographic catheter was placed along the guide wire to search for abnormal blood vessels. After finding abnormal blood vessels, place microcatheters and inject 300/500 µm polyvinyl alcohol foam embolic particles through microcatheters. Finally, the 2.0 and 3.0 mm embolic coils were sent to the abnormal blood vessels through the microcatheter. After the operation, compress the puncture site of the femoral artery for hemostasis and then compress and bind it locally.
For the surgical treatment of patients with tuberculosis-destroyed lung, we mainly adopted the minimally invasive thoracoscopic lung resection method. There were 24 patients in the entire study group who underwent thoracoscopic single-hole minimally invasive surgery. Among them, 4 patients were converted to thoracoscopic-assisted small-incision surgery because of the high risk of adhesion downstream of the endoscope or the difficulty of thoroughly removing the infected lesions. The other 4 cases were treated with traditional thoracotomy because of obvious preoperative thoracic collapse and tuberculous empyema. In all patients, an anterolateral incision was made on the affected side, and the intercostal space was selected for the incision according to the location of the lesion. Generally, the fourth intercostal space is used for upper lobe lung surgery, and the fifth intercostal space is used for middle-lobe and lower-lobe surgery. The observation portal was located in the seventh and eighth intercostal space of the posterior axillary line. The pleural adhesions were gradually separated from the anterior chest wall and mediastinum, and then the diaphragmatic surface adhesions were separated. According to the specific situation, the damaged lung lobe was first separated or the hilar structure was managed first. The treatment of bronchial stump was determined according to the actual situation: bronchial stumps that are far from the infection focus and have light adhesion at the hilum of lung are routinely treated with bronchial occlusion; for patients whose bronchial stump is close to the infection focus or whose thoracic cavity is polluted due to the rupture of the focus during the operation, the bronchial stump was treated with a bronchial occlusion, reinforced with an intermittent 3-0 absorbable line, and then embedded with a pedicled pleura or intercostal muscle flap
Statistical analysis
SPSS23.0 statistical software (IBM Corp.) was used for data processing. The data are expressed as mean ± standard deviation (SD). A t-test was used to analyze the measurement data. The difference was considered statistically significant at P<0.05.
Results
No deaths occurred in the entire study group. There was no significant difference between the 2 groups in general condition and disease condition, including age, duration of disease, location of lesion, or operation method (P>0.05) (Table 1). The operation time in the observation group was significantly shorter than that in the control group (P<0.05), the amount of intraoperative bleeding in the observation group was significantly lower than that in the control group (P<0.05), and the incidence of postoperative complications including pulmonary infection, anemia, and hypoproteinemia in the observation group was significantly lower than that in the control group (P<0.05) (Table 1). Postoperative pulmonary infection occurred in 5 cases, including 1 case without pathogen, 2 cases with Pseudomonas aeruginosa infection, and 2 cases with Acinetobacter baumannii infection. They were cured after consultation with respiratory physicians and adjustment of antibiotic anti-infection treatment. Patients with albumin levels lower than 30 g/L after operation were given symptomatic treatment through intravenous infusion of human serum albumin. Anemic patients with hemoglobin lower than 70 g/L were given intravenous infusion of suspended red blood cells and were instructed to eat a high protein diet. Hypoalbuminemia and anemia were quickly corrected.
Table 1
| Clinical features | Observation group | Control group | P |
|---|---|---|---|
| Age (years) | 56.3±11.1 | 54.7±15.7 | >0.05 |
| Duration of disease (months) | 75.2±20.5 | 62.0±18.6 | >0.05 |
| Location of destroyed lung | |||
| Unilateral whole lung | 2 (15.3) | 2 (13.3) | >0.05 |
| Unilateral lobe | 10 (77.9) | 13 (86.7) | >0.05 |
| Complicated with empyema | 3 (23.1) | 3 (20.0) | >0.05 |
| Surgical manner | |||
| VATS | 9 (69.2) | 11 (73.3) | >0.05 |
| Video-assisted small incision thoracic operation | 2 (15.4) | 2 (13.3) | >0.05 |
| Thoracotomy | 2 (15.4) | 2 (13.3) | >0.05 |
| Blood loss (mL) | 482.3±61.5 | 896.7±104.4 | <0.05 |
| Operation time (min) | 215.0±65.6 | 322.7±69.5 | <0.05 |
| Postoperative complications | |||
| Pulmonary infection | 1 (7.7) | 4 (26.7) | <0.05 |
| Anemia (Hgb <90 g/L) | 1 (7.7) | 5 (33.3) | <0.05 |
| Hypoalbuminemia (Alb <30 g/L) | 2 (15.4) | 6 (40.0) | <0.05 |
Data are shown as number (percentage) or mean ± standard deviation. VATS, video-assisted thoracic surgery; Hgb, hemoglobin; Alb, albumin.
Discussion
Tuberculosis-destroyed lung is one of the manifestations end-stage tuberculosis. Due to extensive pulmonary fibrosis and caseous transformation, the destruction and reconstruction of the lung tissue structure eventually lead to irreversible pathological changes, and it is difficult for antituberculosis drugs to reach the focus to exert effects. Long-term antituberculosis drug treatment is not only ineffective, but also may induce the drug resistance of tuberculosis (6,13), leading to a poor medical treatment effect. For patients with tuberculosis-destroyed lung, it is difficult to remove the tuberculosis bacteria in the focus, and some of these areas will become the source of infection due to bacterial excretion, resulting in the social spread of tuberculosis (14). In addition, patients with tuberculous-damaged lungs are prone to be complicated with mixed infections, including some special infections such as fungal infection (15). This can lead to repeated symptoms of chest distress, asthma, cough, expectoration, and even hemoptysis, resulting in a serious reduction of the patients’ quality of life. Surgical operation has provided the possibility of cure for patients with tuberculosis-destroyed lung and has become the first choice for treatment of tuberculosis-destroyed lung (16).
Surgical treatment of tuberculosis-destroyed lung is difficult. Extensive and dense pleural adhesions limit the operation space and cause substantial difficulties in the exposure of the surgical field of vision (Figure 1). The separation of pleural and hilar adhesions and the hemostasis of the wound further complicate the operation. In addition, pathological changes in the structure of pulmonary alveoli and local bronchi in the diseased lung lead to long-term local inflammatory infiltration, abnormal capillary structure in the diseased lung, extensive formation of collateral circulation outside the diseased lung, and fragile vascular walls (17), which also increase the risk of intraoperative bleeding (18) and the difficulty of hemostasis, and ultimately lead to a longer operation time.
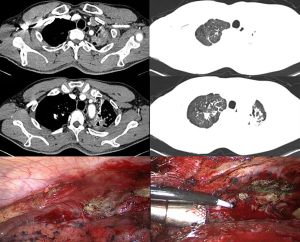
In this study, the operation time of the patients in the control group was 322.7±69.5 min, and the longest case was 450 min and involved considerable bleeding during the operation. The amount of bleeding in the control group reached 896.7±104.4 mL, with 1,550 mL being the most bleeding in a single case. Bai et al. (19) reported 172 patients with tuberculosis-destroyed lung who received surgical treatment, and the blood loss during the operation reached 1,240.0±1,122.5 mL. The long operation time and large amount of bleeding during the operation greatly increase the risk of postoperative complications. In combination with previous treatment experience, our results indicate that patients who have previously undergone embolization of abnormal blood supply arteries in the diseased lungs have less bleeding when undergoing surgical lesion pneumonectomy, and it is relatively easy to stop bleeding, with the operation time being correspondingly shortened, which was clearly reflected in this study.
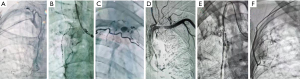
In recent years, with the rapid development of interventional science, angiography and transcatheter arterial occlusion have been widely applied in the examination and treatment of bleeding patients. Among other benefits noted, the arterial occlusion for hemoptysis caused by various reasons has improved year by year (20-22), and these methods can provide effective palliative treatment for hemoptysis patients who cannot tolerate surgical treatment. In our study, 13 patients in the observation group in had hemoptysis symptoms of different degrees before surgery, and all patients were treated with regional systemic artery embolization before surgery to relieve hemoptysis symptoms. The lesions of tuberculous in the lung spread widely, forming close adhesion with intercostal arteries, subclavian vessels, parietal pleura, and other sites (23), with repeated infection, vasodilation, and abundant collateral circulation occurring. During the treatment, as reported in previous literature (24), we also found that all patients in the observation group had blood supply from body arteries other than bronchial arteries (Figure 2). Among them, 11 patients (84.6%) had intercostal artery blood supply, 10 patients (76.9%) had pathological bronchial artery blood supply, 3 patients (23.1%) had internal thoracic artery blood supply, 8 patients (61.5%) had subclavian artery blood supply, and 2 patients (15.4%) had subphrenic artery blood supply. These pathological systemic arterial blood supplies are problematic for operation, significantly increasing intraoperative bleeding and the operation time. Under the guidance of digital subtraction angiography, bleeding vessels or high-risk vessels can be identified, and embolic agent can be injected in advance to embolize them, thus providing good surgical conditions for later surgery and effectively reducing intraoperative bleeding (25). In this study, it is precisely because the patients in the observation group were treated with arterial embolization before surgery, which makes the obvious differences in the observation indicators between the two groups, the amount of bleeding was 896.7±104.4 mL in the control group and 482.3±61.5 mL in the observation group. Moreover, the operation time was significantly decreased, from 322.7±69.5 min in the control group to 215.0±65.6 min in the observation group. This may be related to less bleeding, greater visibility, and relatively easy hemostasis during the operation. In addition, the selective preservation of blood supply in the main bronchus region during the operation did not increase the occurrence of bronchopleural fistula after the operation due to the influence on the blood supply of the bronchial stump. In this study, no bronchopleural fistula occurred in any patients after the operation.
This study has some limitations. First, due to the small sample size, a large-sample, prospective study is needed to further verify the research results. In addition, most of the patients treated with regional systemic artery embolization before surgery had a moderate to severe degree of hemoptysis, while most of the patients in the observation group were patients with a small degree of hemoptysis and no obvious hemoptysis symptoms; thus, our study does not represent a true comparison of patients with different hemoptysis volumes.
Conclusions
The results of this study suggest that regional systemic arterial embolization combined with surgery may effectively reduce intraoperative bleeding, shorten the operation time, and decrease the incidence of postoperative complications in the treatment of tuberculosis-destroyed lung. It is thus a treatment method worthy of further study and discussion.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-224/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-224/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-224/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-224/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Henan Provincial Chest Hospital (No. 20220919) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kabiri EH, Hammoumi ME, Bhairis M, et al. Clinical and surgical analysis of lobectomy for destroyed lobe of the lung: A series of 47 patients. Asian Cardiovasc Thorac Ann 2021;29:772-8. [Crossref] [PubMed]
- Vashakidze SA, Chandrakumaran A, Japaridze M, et al. A case report of persistent drug-sensitive pulmonary tuberculosis after treatment completion. BMC Infect Dis 2022;22:864. [Crossref] [PubMed]
- Koshak YF, Benedykt VV, Prodan AM, et al. Causes of superinfections: deadly bacteria of tuberculosis. Wiad Lek 2022;75:2817-25. [Crossref] [PubMed]
- Ruan H, Gong C, Wang J. The Efficacy and Safety of Surgical Treatment for Patients With Tuberculosis Destroyed Lung With or Without Chronic Pulmonary Aspergillosis. World J Surg 2021;45:1595-601. [Crossref] [PubMed]
- Du W, Zhao Y, Zhang L, et al. Bacteriomes in lesions of pulmonary tuberculosis and its association with status of Mycobacterium tuberculosis excretion. BMC Microbiol 2022;22:280. [Crossref] [PubMed]
- Ghazvini K, Keikha M. The elimination of drug-resistant tuberculosis from a pulmonary resection surgery perspective. Int J Surg 2022;104:106790. [Crossref] [PubMed]
- Bouchikh M, Smahi M, Ouadnouni Y, et al. Pneumonectomy for active and sequelae forms of tuberculosis. Rev Mal Respir 2009;26:505-13. [Crossref] [PubMed]
- Miyano Y, Kanzaki M, Onuki T. Bronchial artery embolization: first-line option for managing massive hemoptysis. Asian Cardiovasc Thorac Ann 2017;25:618-22. [Crossref] [PubMed]
- Seyyedi SR, Tabarsi P, Sadr M, et al. Bronchial Angioembolization for Management of Hemoptysis Due to Pulmonary Tuberculosis. Tanaffos 2021;20:134-9. [PubMed]
- Ruan H, Liu F, Li Y, et al. Long-term follow-up of tuberculosis-destroyed lung patients after surgical treatment. BMC Pulm Med 2022;22:346. [Crossref] [PubMed]
- Hiramatsu M, Atsumi J, Shiraishi Y. Surgical Management of Mycobacterial Infections and Related Complex Pleural Space Problems: From History to Modern Day. Thorac Surg Clin 2022;32:337-48. [Crossref] [PubMed]
- Ruan H, Liu F, Han M, et al. Incidence and risk factors of postoperative complications in patients with tuberculosis-destroyed lung. BMC Pulm Med 2021;21:273. [Crossref] [PubMed]
- Cohen DB, Davies G, Malwafu W, et al. Poor outcomes in recurrent tuberculosis: More than just drug resistance? PLoS One 2019;14:e0215855. [Crossref] [PubMed]
- Deng MH, Zhang SY, Wang Y. Research advances in post-tuberculosis lung disease. ZhonghuaJie He He Hu Xi Za Zhi 2022;45:1041-5. [PubMed]
- Guerra M, Santos N, Miranda J, et al. Surgical management of pulmonary aspergilloma. Rev Port Cir CardiotoracVasc 2008;15:135-8. [PubMed]
- Sayir F, Ocakcioglu I, Şehitoğulları A, et al. Clinical analysis of pneumonectomy for destroyed lung: a retrospective study of 32 patients. Gen Thorac Cardiovasc Surg 2019;67:530-6. [Crossref] [PubMed]
- Han D, Lee HY, Kim K, et al. Burden and clinical characteristics of high gradetuberculosis destroyed lung: a nationwide study. J Thorac Dis 2019;11:4224-33. [Crossref] [PubMed]
- Ruan H, Li Y, Wang Y, et al. Risk factors for respiratory failure after tuberculosis-destroyed lung surgery and increased dyspnea score at 1-year follow-up. J Thorac Dis 2022;14:3737-47. [Crossref] [PubMed]
- Bai L, Hong Z, Gong C, Yan D, Liang Z. Surgical treatment efficacy in 172 cases of tuberculosis-destroyed lungs. Eur J Cardiothorac Surg. 2012;41:335-40. [Crossref] [PubMed]
- Kaufman CS, Kwan SW. Bronchial Artery Embolization. Semin Intervent Radiol 2022;39:210-7. [Crossref] [PubMed]
- Prey B, Francis A, Williams J, et al. Evaluation and Treatment of Massive Hemoptysis. Surg Clin North Am 2022;102:465-81. [Crossref] [PubMed]
- Zheng Z, Zhuang Z, Yang M, et al. Bronchial artery embolization for hemoptysis: A systematic review and meta-analysis. J Interv Med 2021;4:172-80. [Crossref] [PubMed]
- Zhang CJ, Jiang FM, Zuo ZJ, et al. Clinical characteristics and postoperative outcomes of systemic artery-to-pulmonary vessel fistula in hemoptysis patients. Eur Radiol 2022;32:4304-13. [Crossref] [PubMed]
- Nishihara T, Ishikawa H, Omachi N, et al. Prevalence of non-bronchial systemic culprit arteries in patients with hemoptysis with bronchiectasis and chronic pulmonary infection who underwent de novo bronchial artery embolization. Eur Radiol 2022; Epub ahead of print. [Crossref] [PubMed]
- Chen G, Zhong FM, Xu XD, et al. Efficacy of regional arterial embolization before pleuropulmonary resection in 32 patients with tuberculosis-destroyed lung. BMC Pulm Med 2018;18:156. [Crossref] [PubMed]
(English Language Editor: J. Gray)


