
Indwelling pleural catheters for persistent pleural effusions secondary to post lung resection for malignancies
Highlight box
Key findings
• IPC is safe option for recurrent pleural effusion post lung resection.
What is known and what is new?
• Clear indications and guidelines exist for the use of IPC management of malignant pleural effusions and other causes of pleural effusions such as heart failure and hepatic hydrothorax.
• Our article talks about a potential new use for IPC, in the management of recurrent effusions post lung resection.
What is the implication, and what should change now?
• IPC could be used as an alternative to repeated thoracentesis in this subset of patients.
Introduction
Pleural effusions are diagnosed in about 1.5 million individuals in the United States annually (1). Among the causes, pleural infection, heart failure, and malignancy are the most common. Nonmalignant pleural effusions (NMPEs) have a wide variety of etiologies and cause significant morbidity and mortality (2). There are no established guidelines to facilitate management of recurrent NMPE and most management strategies rely on expert experience and data derived from patients with malignancy. Since the majority of patients with NMPE have significant comorbidities, a multidisciplinary approach is often necessary for management.
The clinical impact of an effusion is not merely dependent on volume, but also on fluid localization, rate of development, concomitant effusions and the patient’s general cardiopulmonary condition. Additionally, effusion size is poorly correlated to symptoms such as dyspnea, vertigo and oxygen demand, as well as to symptom relief after drainage (3). Consequently, the threshold of intervention such as indwelling pleural catheter (IPC) or pleurodesis is inconsistent and largely at the discretion of the physician and patients’ symptoms.
Placement of an IPC with intermittent outpatient drainage by the patient or a patient attendant is an accepted treatment for patients with recurrent malignant effusions (4,5). It provides the advantage of shortening length of stay as the procedure can be done in the outpatient setting and can also be used in cases where there is irremediable lung entrapment (6). Other etiologies of NMPE have been successfully treated with an IPC with a low complication rate and high patient satisfaction (7,8). However, to the best of our knowledge, there is no data regarding the use of IPCs for exudative NMPEs post lung resection. We therefore aimed to present our own experience with this management option and determine whether IPC insertion for recurrent NMPE post lung resection is a feasible and safe alternative.
We report our experience of 12 patients with refractory recurrent pleural effusions post lobectomy managed with tunneled IPC. The primary end points were improved symptomatology and successful pleurodesis leading to removal of catheter. Secondary outcomes were complications after indwelling catheter placement including infection, dislodgement, pain and bleeding. We present this article in accordance with the AME Case Series reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1517/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics review board of the University of Florida (IRB202200492) and individual consent for this retrospective analysis was waived. Cases were non-consecutive. All patients were treated at the University of Florida, a large tertiary academic center in Gainesville, Florida. Patients who underwent lobectomy or segmentectomy as part of the treatment plan for lung cancer between January 2019 and June 2022 were identified. Treatment plans were decided by a multidisciplinary tumor board and patients received appropriate adjuvant therapy when indicated, electronic medical records for these patients were screened for post-surgical pleural effusion. A total of 422 patients had lung resection during this time. Thirty-eight of those patients had postoperative symptomatic pleural effusion requiring drainage within the first 90 days post-surgery. All the effusions were exudative in nature and assumed nonmalignant given negative pleural cytology (PET scan was not used as part of the protocol). Eighteen patients had the effusion drained at least twice and 12 had recurrent effusion after second thoracentesis and underwent IPC placement. Figure 1 shows schematic representation of patient screening. All subjects were required to have undergone at least two therapeutic thoracentesis and have evidence of recurrent pleural effusion prior to consideration for IPC placement. Data on patient’s baseline demographics, underlying cancer and stage, type of surgery, catheter side, complications, time of catheter removal, need of subsequent procedures and day of last follow-up were collected. All catheters (PleurX, CareFusion, San Diego, California, USA) were placed in the bronchoscopy unit under moderate sedation by an interventional pulmonologist using ultrasound guidance. Tunneled IPCs were placed in ambulatory setting and patients were discharged on the same day of the procedure. Vacuum bottles were used for drainage. Patients caregivers were trained on the day of procedure along with the patient to assist with catheter drainage. Strict drainage protocol was followed with drainage every day till the output went below 500 mL daily. At that point the drainage was switched to every other day. When the fluid drainage went below 50 mL during drainage consistently for 2–3 weeks patients were reassessed with a chest X-ray and if there was no evidence of fluid re-accumulation, patients were scheduled for catheter removal.
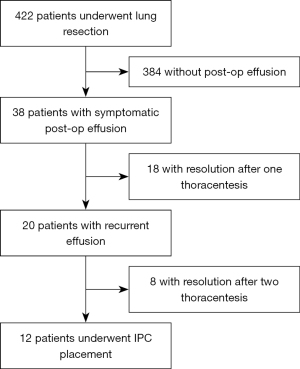
The primary end points were spontaneous pleurodesis (SP) and improvement in symptoms. Other outcomes included lung re-expansion, IPC removal, pleural effusion recurrence and need for subsequent pleural intervention. Pleurodesis was defined as successful removal of IPC with no further pleural intervention needed after catheter removal. All patients were followed for at least 3 months post catheter removal.
The index procedure was identified as the initial IPC placement. Adequate lung re-expansion was defined as post procedural resolution of pleural effusion on follow up imaging. Recurrence of effusion was defined as re-accumulation of pleural fluid on follow up surveillance CT scans. Post procedural complications were also assessed including infection, dislodgement, occlusion requiring tissue plasminogen activator (tPA), bleeding, tumor seeding, pain requiring removal, transient respiratory distress.
Statistical analysis
We used SPSS statistical software version 20.0.1.1 (IBM Corp., Armonk, NT, USA) for quantitative statistics. Analysis was conducted with the patient as the unit of analysis, quantitative data was reported as median with standard deviation (SD).
Results
There was a total of 12 IPCs inserted in 12 patients. The median age at the time of index procedure was 68.5 years SD 9.6. Seven patients were female (58%), and five patients were male (42%), all patients had exudative effusions according to Light’s criteria, all twelve patients reported shortness of breath prior to therapeutic thoracentesis and symptomatic relief after pleural fluid was drained. Every patient underwent at least two therapeutic thoracentesis prior to IPC insertion and three patients (25%) had 3 thoracentesis. Seven patients (58.3%) had the IPC placed on the right hemithorax and five (41.7%) were placed on the left hemithorax. All effusions were nonmalignant on repeated cytology.
The most common type of cancer was lung adenocarcinoma accounting for 58% of patients, followed by squamous cell carcinoma with 25%, atypical carcinoid was the least common type of malignancy with 17%. Most patients underwent lobectomy only, 2 patients (17%) underwent additional lobe segmentectomy at the time of surgery, staging, demographic and outcomes data is described in Table 1.
Table 1
| Patient | Age (years) | Sex | Staging | Length of IPC (days) | Spontaneous pleurodesis | Symptomatic relief | Complications |
|---|---|---|---|---|---|---|---|
| 1 | 70 | Female | T1b (IA2) | 77 | Yes | Yes | Pain |
| 2 | 72 | Female | T2aN0 (IB) | 68 | Yes | Yes | None |
| 3 | 63 | Female | T2aN0 (IB) | 106 | Yes | Yes | Pain, catheter occlusion |
| 4 | 76 | Male | T2aN1 (IIA) | 84 | Yes | Yes | Pain, cellulitis |
| 5 | 81 | Male | T1cN0 (IA3) | 73 | Yes | Yes | None |
| 6 | 77 | Female | T2aN0 (IB) | 56 | Yes | Yes | Pain |
| 7 | 73 | Male | RLL: T1bN0 (IA2); RUL: T1cN0 (IA3) |
47 | Yes | Yes | None |
| 8 | 68 | Male | T3N0 (IIB) | 82 | Yes | Yes | Pain |
| 9 | 49 | Female | T4N0 (IIIA) | 102 | Yes | Yes | Catheter occlusion |
| 10 | 52 | Female | T3N1 (IIIA) | 44 | Yes | Yes | Pain |
| 11 | 71 | Male | T4N1 (IIIB) | 71 | Yes | Yes | Pain, cellulitis |
| 12 | 70 | Female | T3N0 (IIB) | 123 | Yes | Yes | Pain, catheter occlusion |
RLL, right lower lobe; RUL, right upper lobe; IPC, indwelling pleural catheter.
Mean time from day of resection to first and second thoracentesis was 26.1 days (SD 19.4) and 52.1 days (SD 32.8) respectively. Mean time to index procedure was 78.4 days post-surgery. The mean length of IPC catheter was 77.7 days SD 23.8. All 12 patients achieved SP, there was no second pleural intervention or re-accumulation of fluid on follow up imaging in any of the subjects after IPC removal.
There were a total of eight patients (66.6%) that reported pain after the index procedure which was managed conservatively with oral pain medications. Three patients (25%) had occlusion of the IPC that was successfully managed with instillation of fibrinolytics. Two patients (16.7%) had skin infection related to catheter placement that was managed with oral antibiotics and IPC was kept in place, there were no cases of pleural infections that required catheter removal. Table 1 shows outcomes and complications for each patient.
Discussion
Persistent pleural effusions can be observed after lung resection due to disorders in the pleural fluid balance and reduced postoperative lung expansion. Based on few series, most of the patients who undergo lobectomy won’t develop pleural effusions and the incidence of post lobectomy symptomatic pleural effusion requiring intervention after discharge is around 1–4% (9-11) even when chest tubes are removed with output of more than 400 mL per day. Various etiologies of recurrent pleural effusion post lung resection include infection, chylothorax, hemothorax, malignancy vs. exudative nonmalignant effusion (12).
The goal of IPC in the settings of recurrent effusions is to allow for outpatient management of symptomatic pleural effusions with potential for SP or bridge to definitive treatment. SP is defined by most as removal of IPC without further intervention and without re-accumulation of symptomatic effusion after its removal (13), can be anticipated in 45% of patients with malignant effusions (14). Although the mechanism is not known, SP is hypothesized to be due to an inflammatory process initiated by the presence of a foreign body within the pleural space (15). The rate of SP after IPC in nonmalignant disease is uncertain and reported rates vary widely from 29% to 71% (8,16,17). The variation is likely in part due to the heterogenous etiologies and small numbers reported. Management of NMPE with repeat thoracentesis or pleurodesis remains to be compared with the use of IPCs especially in this subset of patients with effusion post lung resection.
Data from our cohort of patients shows that management of pleural effusions after lung resection with IPC is effective not only in relieving shortness of breath associated with recurrent effusions but as a tool to achieve SP which was achieved in all patients, this is higher than the rate of SP outlined in previous series where only 44% of patients had SP with non-malignant pleural effusion (8). As with previous studies, use of IPC’s resulted in less need for repeated pleural interventions (18,19). Non-serious infectious complications (cellulitis) were similar to other reported series of NMPE (20). Although no patients in this cohort had pleural space infection.
Our study has several limitations. It is a small series with only 12 patients. Patients were evaluated in retrospective fashion which is subject to selection and treatment bias. It is also a single center study and may not be generalizable to other institutions. Due to the retrospective character of our study, palliation could only be assessed in an indirect manner focusing on the need of additional (invasive) procedures like thoracenteses or (second) chest tubes. We however reviewed every patient admitted with lobectomy for lung cancer and all of them with pleural effusion were followed by a single pulmonary provider with standard protocol decreasing the likelihood of selection bias. Our study did show that IPC for post-surgical pleural effusion is highly effective, well tolerated and with good success rates for pleurodesis.
Conclusions
Although conclusions from this study are not generalizable given small sample and lack of a control group, treatment with IPCs for pleural effusions post lung resection seems favorable in achieving pleurodesis and symptomatic relief of dyspnea with similar rates of infection as other studies with IPCs in non-malignant effusions. More studies are needed to compare different methods of treatment and assessment. Further studies, especially other studies comparing IPC to serial thoracentesis should be done to ascertain these results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1517/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1517/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1517/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1517/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics review board of the University of Florida (IRB202200492) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Light RW. Pleural diseases. 6th ed 2013.
- DeBiasi EM, Pisani MA, Murphy TE, et al. Mortality among patients with pleural effusion undergoing thoracentesis. Eur Respir J 2015;46:495-502. [Crossref] [PubMed]
- Thomas R, Jenkins S, Eastwood PR, et al. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 2015;21:338-45. [Crossref] [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Warren WH, Kalimi R, Khodadadian LM, et al. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008;85:1049-55. [Crossref] [PubMed]
- Pien GW, Gant MJ, Washam CL, et al. Use of an implantable pleural catheter for trapped lung syndrome in patients with malignant pleural effusion. Chest 2001;119:1641-6. [Crossref] [PubMed]
- Chalhoub M, Harris K, Castellano M, et al. The use of the PleurX catheter in the management of non-malignant pleural effusions. Chron Respir Dis 2011;8:185-91. [Crossref] [PubMed]
- Bhatnagar R, Reid ED, Corcoran JP, et al. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax 2014;69:959-61. [Crossref] [PubMed]
- Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg 2014;45:241-6. [Crossref] [PubMed]
- McKenna RJ Jr, Mahtabifard A, Pickens A, et al. Fast-tracking after video-assisted thoracoscopic surgery lobectomy, segmentectomy, and pneumonectomy. Ann Thorac Surg 2007;84:1663-7; discussion 1667-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [Crossref] [PubMed]
- Sziklavari Z, Neu R, Hofmann HS, et al. Persistent pleural effusion following thoracic surgery. Chirurg 2015;86:432-6. [Crossref] [PubMed]
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762-7. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Warren WH, Kim AW, Liptay MJ. Identification of clinical factors predicting Pleurx catheter removal in patients treated for malignant pleural effusion. Eur J Cardiothorac Surg 2008;33:89-94. [Crossref] [PubMed]
- Bintcliffe O, Arnold D, Maskell N. Indwelling pleural catheters for benign pleural effusions. Current Respiratory Care Reports 2014;3:61-70. [Crossref]
- Srour N, Potechin R, Amjadi K. Use of indwelling pleural catheters for cardiogenic pleural effusions. Chest 2013;144:1603-8. [Crossref] [PubMed]
- Iyer NP, Reddy CB, Wahidi MM, et al. Indwelling Pleural Catheter versus Pleurodesis for Malignant Pleural Effusions. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2019;16:124-31. [Crossref] [PubMed]
- Boshuizen RC, Vd Noort V, Burgers JA, et al. A randomized controlled trial comparing indwelling pleural catheters with talc pleurodesis (NVALT-14). Lung Cancer 2017;108:9-14. [Crossref] [PubMed]
- Chen A, Massoni J, Jung D, et al. Indwelling Tunneled Pleural Catheters for the Management of Hepatic Hydrothorax. A Pilot Study. Ann Am Thorac Soc 2016;13:862-6. [Crossref] [PubMed]


