
Increased TIM-3 expression in tumor-associated macrophages predicts a poorer prognosis in non-small cell lung cancer: a retrospective cohort study
Highlight box
Key findings
• Our results demonstrated that high TIM-3 expression in TAMs was an independent predictor of worse prognosis in patients.
What is known and what is new?
• TIM-3, as a negative regulatory immune checkpoint, is an important tumor immune checkpoint.
• High TIM-3 expression levels in TAMs are negatively correlated with OS.
What is the implication, and what should change now?
• TIM-3 expression in TAMs and clinicopathological features of patients have prognostic value in NSCLC.
Introduction
Lung cancer is one of the most common malignant tumors, and has become the leading cause of cancer-related death in both men and women worldwide (1). Non-small cell lung cancer (NSCLC) accounts for approximately 80–85% of all lung cancers (1,2). With the development of molecular biomarkers, non-small cell lung cancer has made major breakthroughs in immunotherapy and targeted therapy. However, the prognostic biomarkers of NSCLC are still very limited. Biomarkers represented by PD-L1 expression and tumor mutational burden (TMB) can be used to assess the prognosis of patients, but their predictive value is also controversial due to the need for sufficient tumor tissue. Clinical biomarkers should be considered not only in practicability, but also in operability and high cost (3). In addition to stage of the disease, mutated genes such as EGFR, KRAS have been validated as prognostic biomarkers in NSCLC. But obtaining such genetic information requires invasive procedures, and high sequencing costs (4). Cellular senescence has recently been promoted as an emerging hallmark of cancer. However, little is known about the consequence of cellular senescence on prognosis and survival in distinct types of cancer. In NSCLC, only two previous reports demonstrated a negative prognostic value of individual immunohistochemical senescence markers, such as lipofuscin accumulation (5) and high p21WAF1/Cip1 and high Ki67 (6), however, both in rather histological heterogenous patient populations. Therefore, we need prognostic biomarkers of NSCLC that can take into account cost, operability and practicality. For a long time, studies have focused on tumor cells themselves. In recent years, the perspective of researchers has gradually expanded from the tumor cells themselves to their microenvironment. The tumor microenvironment (TME) contains tumor cells, immune cells, and stromal cells (7). Tumor-associated macrophages (TAMs), the most abundant immune cells in the TME (8), play an important role in the development of NSCLC and may be potential prognostic markers for the prediction of NSCLC (9). Therefore, exploring new immunomodulatory molecules associated with TAMs is important for identifying effective therapeutic strategies for NSCLC.
The T-cell immunoglobulin and mucin domain-containing molecule (TIM) gene family was discovered in 2001; it plays an important role in the regulation of immune function (10,11). The human TIM family comprises 3 genes: TIM-1, TIM-3, and TIM-4. TIM-3 is expressed in a variety of immune cells, including T cells (12), natural killer cells (NK) (13), dendritic cells (DCs) (14), and macrophages (15). TIM-3 has been recognized as a key negative regulator in T cell-mediated responses (16), and it has been reported that its expression in tumor cells, tumor-infiltrating lymphocytes, or both, is negatively correlated with overall survival (OS) of patients with lung adenocarcinoma (17). Additionally, it has been demonstrated that TIM-3 upregulation in TAMs is strongly correlated with the poor survival of patients with hepatocellular carcinoma (HCC), and that TIM-3 has a protumoral effect on TAMs in HCC (18), which suggests that its expression in TAMs could be involved in tumorigenesis. Predicting the clinical outcome of patients with advanced NSCLC through the level of TIM3 will provide new guiding ideas for clinical practice. In this study, we explored the association between TIM-3 expression in TAMs and clinicopathological features of patients and its prognostic value in NSCLC. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-227/rc).
Methods
Patients
Patients with NSCLC who had undergone surgery at Zhoushan Hospital (Zhoushan, Zhejiang, China) between January 2010 and January 2013 were included in this study. Clinicopathological features, such as age, sex, smoking, carcinoembryonic antigen (CEA), and tumor size, were obtained from clinical records. Histopathological diagnoses were made according to the World Health Organization classifications. The tumor-node-metastasis (TNM) classification of patients was determined according to the 8th edition of the Union for International Cancer Control (19). We measured OS from the date of surgery to the date of death due to NSCLC. The inclusion criteria were as follows: (I) pathologically confirmed primary NSCLC and (II) complete data on clinicopathological features. The exclusion criteria were as follows: (I) patients who received preoperative chemotherapy or other treatment and (II) patients with severe chronic diseases, other tumors, and autoimmune diseases. A total of 248 patients with NSCLC were included in this study. The subtypes of NSCLC included 155 adenocarcinomas and 93 squamous cell carcinomas. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Zhoushan Hospital (No. 2018-146). Individual consent for this retrospective analysis was waived.
Immunohistochemistry and evaluations
A total of 4 serial 4-µm-thick formalin-fixed and paraffin-embedded tissue sections were cut from each NSCLC specimen. They were dewaxed in xylene, hydrated with a series of anhydrous ethanol, and then repaired by microwave. After washing with phosphate buffered saline (PBS) to reduce specific binding, the slide was sealed with goat serum blocking solution and the excess liquid was poured. Add the first antibody in proportion, that is, use the mouse anti-human CD68 monoclonal antibody (Abcam, ab125212), mouse anti-human CD163 monoclonal antibody (Abcam, ab87099), and mouse anti-human tim-3 monoclonal antibody (Abcam, ab185703), use PBS instead of the first antibody as the negative control, and then incubate at 4 ℃ overnight. After tissue sections were washed with PBS, EnVision horseradish peroxidase (HRP; Conway China) was used for secondary antibody culture. Diaminobenzidine (DAB) was used for color development, and the sections were stained with hematoxylin. Tumor stroma was defined as the area where tumor stromal cells accounted for more than 70% of the total cells. All slides were reviewed by 2 experienced pathologists who were blinded to the clinical records, and the evaluation of immunohistochemistry (IHC) was based on consensus. The number of CD68- or CD163-positive cells in the tumor stroma was separately counted in 5 representative fields (×400 magnification) using a pathology digital imaging system (Nanozoomer Virtual Slide System; Hamamatsu Photonics, Shizuoka, Japan). The average number of cells in the 5 fields represented the number of CD68- or CD163-positive cells for each NSCLC section. TIM-3 staining was scored separately for TAMs in the tumor stroma according to a previous study (14), and graded as follows: 0, 0–5%; 1, 5–25%; 2, 25–50% and 3, >50%. A score of 0 or 1 was considered to indicate “low expression”, and score 2 or 3 was considered to indicate “high expression”.
Statistical analysis
The software SPSS 17.0 (IBM Corp, Armonk, NY, USA) was used for data analysis. The chi-square test was used for univariate analysis to evaluate the correlation between TIM-3 expression in TAMs and clinicopathological features of NSCLC. Receiver operating characteristic (ROC) analysis was performed to confirm the optimal cutoff value of TIM-3-positive expression. Spearman rank correlation was used to analyze the correlation between TIM-3, CD68, and CD163. The log-rank test was used to estimate the postoperative OS. The Cox proportional hazard model was used for univariate and multivariate analyses to assess the association between TIM-3 and postoperative OS. All statistical tests were 2-sided, and a P value <0.05 was considered statistically significant.
Results
TIM-3 expression in TAMs in the tumor stroma
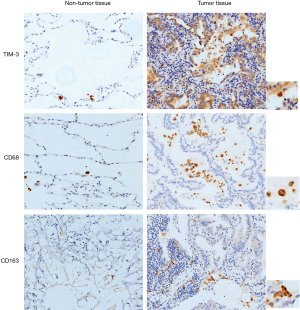
Representative fields on NSCLC slides stained for CD68, CD163, and TIM-3 are displayed in Figure 1. CD68, a macrophage marker, was detected in the tumor stroma of NSCLC specimens. CD163, an M2 macrophage marker, was also detected in the tumor stroma and frequently co-localized with stromal CD68+ macrophages. Strong correlations were observed between the distributions of CD68+ macrophages and CD163+ macrophages in the tumor stroma (r=0.7380, P<0.01). TIM-3 expression was also frequently co-localized with CD68+ macrophages or CD163+ macrophages in the tumor stroma of NSCLC specimens. Using a 25% threshold, high TIM-3 expression was detected in 146 (58.87%) of the NSCLC samples and 88 (56.77%) of the adenocarcinoma samples. Additionally, in tumor stroma, CD68+ macrophages [TIM-3, odds ratio (OR) =2.17, 95% confidence interval (CI): 1.08–3.91, P=0.026] and CD163+ macrophages (TIM-3, OR=2.69, 95% CI: 1.48–4.45, P=0.015) were independent predictive factors for TIM-3 expression.
Correlations between TIM-3 expression in TAMs and the clinicopathological features of patients with NSCLC and adenocarcinoma
As shown in Table 1, high TIM-3 expression in TAMs was more frequently identified in patients with higher CEA levels (>5 ng/mL; TIM-3, 64.29% vs. 35.71%, P=0.048), lymph node metastasis (positive; TIM-3, 67.54% vs.32.46%, P=0.010), high CD68 expression (TIM-3, 65.89% vs. 34.11%, P=0.018), and high CD163 expression (TIM-3, 67.79% vs. 32.21%, P<0.001). No statistically significant differences were found in the association between TIM-3 expression and age, sex, smoking, tumor size, histology type, pleural invasion, or stage.
Table 1
| Variables | TIM-3 expression, n (%) | P value | |
|---|---|---|---|
| Low (n=102) | High (n=146) | ||
| Age (years) | 0.636 | ||
| ≤65 | 57 (39.86) | 86 (60.14) | |
| >65 | 45 (42.86) | 60 (57.24) | |
| Gender | 0.927 | ||
| Male | 49 (40.83) | 71 (59.17) | |
| Female | 53 (41.41) | 75 (58.59) | |
| Smoking | 0.735 | ||
| Non-smoker | 60 (40.27) | 89 (59.73) | |
| Smoker | 42 (42.42) | 57 (57.58) | |
| CEA, ng/mL | 0.048 | ||
| ≤5 | 52 (49.06) | 56 (51.85) | |
| >5 | 50 (35.71) | 90 (64.29) | |
| Tumor size, cm | 0.127 | ||
| ≤3 | 41 (35.96) | 73 (64.04) | |
| >3 | 61 (45.52) | 73 (54.48) | |
| Histology type | 0.386 | ||
| Ad | 67 (43.23) | 88 (56.77) | |
| Sq | 35 (37.64) | 58 (62.37) | |
| PI | 0.066 | ||
| Absent | 61 (46.56) | 70 (53.44) | |
| Present | 41 (35.04) | 76 (64.96) | |
| LN | 0.010 | ||
| Negative | 65 (48.51) | 69 (51.49) | |
| Positive | 37 (32.46) | 77 (67.54) | |
| Stage | 0.125 | ||
| I–IIIa | 74 (45.12) | 90 (54.88) | |
| IIIb–IV | 28 (33.33) | 56 (66.67) | |
| Grade | 0.269 | ||
| G1–G2 | 66 (44.90) | 81 (55.10) | |
| G3–G4 | 36 (35.64) | 65 (64.36) | |
| CD68 expression | 0.018 | ||
| Low | 58 (48.74) | 61 (51.26) | |
| High | 44 (34.11) | 85 (65.89) | |
| CD163 | <0.001 | ||
| Low | 54 (54.55) | 45 (45.45) | |
| High | 48 (32.21) | 101 (67.79) | |
TIM-3 expression is defined as TAM-positive. NSCLC, non-small cell lung cancer; CEA, carcinoembryonic antigen; Ad, adenocarcinoma; Sq, squamous cell carcinoma; LN, lymph node metastasis; PI, pleural invasion; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; TAM, tumor-associated macrophage.
Subsequently, we explored the correlations between TIM-3 expression in TAMs and the clinicopathological features of adenocarcinomas. As shown in Table 2, our results showed that TIM-3 expression in TAMs was more frequently observed in patients with invasive adenocarcinoma (TIM-3, 54.29% vs. 45.71%, P=0.018), lymph node metastasis (positive: TIM-3, 53.16% vs. 46.84%, P=0.017), high CD68 expression (TIM-3, 61.11% vs. 38.89%, P=0.019), and high CD163 expression (TIM-3, 52.56% vs. 47.44%, P=0.028). There were no statistically significant differences between TIM-3 expression and age, sex, smoking, CEA level, tumor size, pleural invasion, or grade.
Table 2
| Variables | TIM-3 expression, n (%) | P value | |
|---|---|---|---|
| Low (n=87) | High (n=68) | ||
| Age (years) | 0.564 | ||
| ≤65 | 42 (53.85) | 36 (46.15) | |
| >65 | 45 (58.44) | 32 (41.56) | |
| Gender | 0.211 | ||
| Male | 36 (50.70) | 35 (49.30) | |
| Female | 51 (60.71) | 33 (39.29) | |
| Smoking | 0.316 | ||
| Non-smoker | 48 (60.00) | 32 (40.00) | |
| Smoker | 39 (52.00) | 36 (48.00) | |
| CEA, ng/mL | 0.752 | ||
| ≤5 | 40 (54.79) | 33 (45.21) | |
| >5 | 47 (57.32) | 35 (42.68) | |
| Tumor size, cm | 0.225 | ||
| ≤3 | 52 (60.47) | 34 (39.53) | |
| >3 | 35 (50.72) | 34 (49.28) | |
| Histology type | 0.018 | ||
| AIS/MIA | 55 (64.71) | 30 (35.29) | |
| IAC | 32 (45.71) | 38 (54.29) | |
| PI | 0.354 | ||
| Absent | 50 (59.52) | 34 (40.48) | |
| Present | 37 (52.11) | 34 (47.89) | |
| LN | 0.017 | ||
| Negative | 50 (65.79) | 26 (34.21) | |
| Positive | 37 (46.84) | 42 (53.16) | |
| Stage | 0.221 | ||
| Ia | 54 (61.36) | 36 (38.64) | |
| Ib–IV | 33 (50.77) | 32 (49.23) | |
| Grade | 0.573 | ||
| G1 | 50 (58.14) | 36 (41.86) | |
| G2–G4 | 37 (53.62) | 32 (46.38) | |
| CD68 expression | 0.019 | ||
| Low | 51 (75.00) | 27 (25.00) | |
| High | 36 (38.89) | 41 (61.11) | |
| CD163 | 0.028 | ||
| Low | 50 (64.94) | 27 (35.06) | |
| High | 37 (47.44) | 41 (52.56) | |
TIM-3 expression is defined as TAM-positive. CEA, carcinoembryonic antigen; AIS, adenocariconoma in situ; MIA, microinvasive adenocariconoma; IAC, invasive adenocariconoma; PI, pleural invasion; LN, lymph node metastasis; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; TAM, tumor-associated macrophage.
Correlations between TIM-3 expression in TAMs and the clinical outcomes of NSCLC and lung adenocarcinoma
In NSCLC, the relationship between TIM-3 expression in TAMs and prognosis was analyzed. Our results showed that the OS of the high TIM-3 expression groups (35.33±24.91) was shorter than that of the low TIM-3 expression groups (60.28±16.89) (TIM-3, P=0.01) (Figure 2A). To further stratify patients into subgroups, the combination of TIM-3 and/or CD68/CD163 expression in TAMs was used to explore the relationship with prognosis. As shown in Figure 2B, patients with high TIM-3 and high CD68/CD163 expression levels had the worst prognoses; patients with high expression levels of either TIM-3 or CD68/CD163 had moderate prognoses, and patients with low expression levels of both TIM-3 and CD68/CD163 had the best prognoses (P<0.05). Further multivariate analyses demonstrated that larger tumor size [hazard ratio (HR): 2.33, 95% CI: 1.51–3.88, P=0.035], lymph node metastasis (HR: 2.68, 95% CI: 1.47–3.98, P=0.027), high CD68 expression (HR: 1.91, 95% CI: 1.11–3.23, P=0.037), high CD163 expression (HR: 2.12, 95% CI: 1.14–3.40, P=0.024), and high TIM-3 expression (HR: 3.43, 95% CI: 2.02–5.83, P=0.019) were independent prognostic factors of worse OS rates (Table 3).
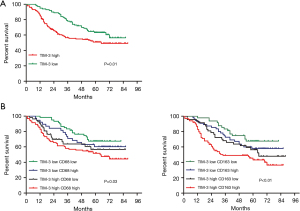
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (>65 vs. ≤65 years) | 1.10 | 0.78–1.64 | 0.531 | ||||
| Gender (male vs. female) | 1.03 | 0.72–1.50 | 0.622 | ||||
| Smoking (smoker vs. never-smoker) | 1.05 | 0.74–1.55 | 0.784 | ||||
| CEA (>5 vs. ≤5 ng/mL) | 1.20 | 0.82–1.72 | 0.073 | ||||
| Tumor size (>3 vs. ≤3 cm) | 3.54 | 2.19–5.86 | 0.012 | 2.33 | 1.51–3.88 | 0.035 | |
| Histology type (Sq vs. Ad) | 1.09 | 0.76–1.60 | 0.454 | ||||
| PI (present vs. absent) | 1.42 | 0.98–2.17 | 0.323 | ||||
| LN (present vs. absent) | 4.35 | 2.44–10.23 | 0.016 | 2.68 | 1.47–3.98 | 0.027 | |
| Stage (Ib-IV vs. Ia) | 1.81 | 1.12–3.20 | 0.028 | 1.13 | 0.67–1.90 | 0.080 | |
| Grade (G2-G4 vs. G1) | 1.60 | 1.09–2.31 | 0.032 | 1.02 | 0.65–1.49 | 0.076 | |
| CD68 expression (high vs. low) | 1.74 | 1.02–3.16 | 0.011 | 1.91 | 1.11–3.23 | 0.037 | |
| CD163 expression (high vs. low) | 1.88 | 1.08–3.24 | 0.014 | 2.12 | 1.14–3.40 | 0.024 | |
| TIM-3 expression (high vs. low) | 2.97 | 1.96–4.75 | 0.008 | 3.43 | 2.02–5.83 | 0.019 | |
TIM-3 expression is defined as TAM-positive. NSCLC, non-small cell lung cancer; CEA, carcinoembryonic antigen; Ad, adenocarcinoma; Sq, squamous cell carcinoma; PI, pleural invasion; LN, lymph node metastasis; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; HR, hazard ratio; CI, confidence interval.
In lung adenocarcinoma, our results also indicated that the OS of the high TIM-3 expression groups (40.70±25.88) was shorter than that of the low TIM-3 expression groups (59.80±14.37) (TIM-3, P=0.03) (Figure 3A). Patients with high TIM-3 and high CD68/CD163 expression had the worst prognosis; patients with low expression of both TIM-3 and CD68/CD163 expression had the best prognosis (P<0.05) (Figure 3B). Further multivariate analyses confirmed that larger tumor size (HR: 2.50, 95% CI: 1.59–3.93, P=0.039), invasive adenocarcinoma (HR: 3.40, 95% CI: 2.16–5.80, P=0.007), lymph node metastasis (HR: 2.70, 95% CI: 1.63–4.50, P=0.033), high CD68 expression (HR: 1.65, 95% CI: 1.11–2.38, P=0.025), high CD163 expression (HR: 1.97, 95% CI: 1.15–3.82, P=0.019), and high TIM-3 expression (HR: 2.76, 95% CI: 1.68–4.61, P=0.015) were independent prognostic factors worse OS in lung adenocarcinoma (Table 4).
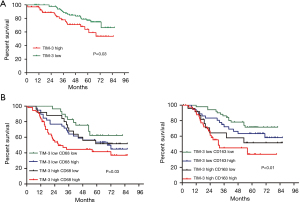
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (>65 vs. ≤65 years) | 1.06 | 0.71–1.57 | 0.823 | ||||
| Gender (male vs. female) | 1.08 | 0.71–1.60 | 0.716 | ||||
| Smoking (smoker vs. never-smoker) | 1.01 | 0.70–1.40 | 0.835 | ||||
| CEA (>5 vs. ≤5 ng/mL) | 1.50 | 1.04–2.22 | 0.089 | ||||
| Tumor size (>3 vs. ≤3 cm) | 3.01 | 2.02–4.98 | 0.017 | 2.50 | 1.59–3.93 | 0.039 | |
| Histology type (IAC vs. AIS/MIA) | 4.16 | 2.67–6.89 | 0.006 | 3.40 | 2.16–5.80 | 0.007 | |
| PI (present vs. absent) | 1.62 | 1.10–2.36 | 0.059 | ||||
| LN (present vs. absent) | 3.28 | 2.11–5.67 | 0.027 | 2.70 | 1.63–4.50 | 0.033 | |
| Stage (Ib-IV vs. Ia) | 1.70 | 1.14–2.53 | 0.036 | 1.43 | 0.92–2.30 | 0.042 | |
| Grade (G2-G4 vs. G1) | 1.65 | 1.10–2.45 | 0.038 | 1.51 | 1.04–2.22 | 0.044 | |
| CD68 expression (high vs. low) | 1.87 | 1.08–3.22 | 0.014 | 1.65 | 1.11–2.38 | 0.025 | |
| CD163 expression (high vs. low) | 2.03 | 1.16–3.94 | 0.015 | 1.97 | 1.15–3.82 | 0.019 | |
| TIM-3 expression (high vs. low) | 3.05 | 2.11–5.10 | 0.007 | 2.76 | 1.68–4.61 | 0.015 | |
TIM-3 expression is defined as TAM-positive. CEA, carcinoembryonic antigen; IAC, invasive adenocariconoma; AIS, adenocariconoma in situ; MIA, microinvasive adenocariconoma; PI, pleural invasion; LN, lymph node metastasis; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; TAM, tumor-associated macrophage; HR, hazard ratio; CI, confidence interval.
Discussion
To the best of our knowledge, this is the first study to explore TIM-3 expression in TAMs in tumor stroma and its prognostic value in patients with NSCLC. Our study found that high TIM-3 expression was positively correlated with high CD68/CD163 TAM density. Moreover, high TIM-3 expression levels in TAMs were negatively correlated with worse OS. The combination of high TIM-3 and/or CD68/CD163 TAM density could further stratify patients into different subgroups with different prognoses. Our results demonstrated that high TIM-3 expression in TAMs was an independent predictor of worse prognosis in patients. A previous study (20) found by analyzing the expression of TIM-3 protein on NSCLC tumor cells and tumor infiltrating lymphocytes (TILs). High level of TIM-3 on TILs indicated shorter recurrence-free survival (RFS) and overall survival (OS) (RFS 1.800 years, 95% CI: 1.230–2.370 vs. 0.870 years, 95% CI: 0.212–1.528, P=0.048) (OS 2.960 years, 95% CI: 2.268–3.652 vs. 1.080 years, 95% CI: 0.228–1.932, P=0.034). Their research proved that TIM-3 was expressed on all NSCLC tumor cells and TILs of NSCLC pathological types. NSCLC patients with high TIM-3 level on TIL have poor prognosis. This study can also prove the feasibility of our research from the side.
A previous study has shown that TAMs are the most populous cell type of the TME in limited-stage SCLC, especially in tumor nests, where their cellular density far exceeds the number of T-cells. Most CD68+ TAMs are M2-polarised, and express CD163 in neuroendocrine (NE)-low tumor subsets, creating an immunosuppressive microenvironment peculiarly inside tumor nests (21). In addition, a previous study has confirmed that the presence of CD68+ TAMs in tumor nests was significantly detrimental to OS (22). These studies related to small cell lung cancer strongly support our conclusions. We have reason to believe that similar immune mechanisms play an indispensable role in both types of cancer. This will also become the focus of our next research.
TIM-3 was initially found to be highly expressed in terminally differentiated T helper 1 cells (23). In recent years, it has been found that TIM-3 is highly expressed in a variety of immune cells, including DCs, monocytes/macrophages, and NK cells, and is also expressed in tumor cells, such as in gastric cancer, liver cancer, melanoma, and B cell lymphoma (24). The inhibitory function of TIM-3 in the immune system has been found to be associated with the development of multiple tumors (25). TIM-3 is regarded as an important immune checkpoint and its expression has been explored in the TME (24-26). It has been well-documented that TIM-3 expression is significantly upregulated in tumor infiltrating immune cells, and is associated with poor prognosis of patients with cancer (17,27). Studies have demonstrated that TIM-3 expression is increased in tumor infiltrating CD8+ T cells or TAMs, and predicts poor prognosis in patients with HCC (18,28). In mouse liver cancer models, TGF-beta type 1 factor (TGF-β) can promote the expression of TIM-3 on tumor-associated macrophages (TAMs) in HCC, and TIM-3 inhibits the activation of tumor-specific CD8+ T cells. The study has found that TGF-β can induce the expression of the TIM-3 ligand Gal-9 when tumor cell growth undergoes a hypoxic phase; in the normal oxygen availability phase, TGF-β induces its own expression through the Smad3 transcription factor, and the Smad3 transcription factor, and induces the expression of Gal-9. In these two different stages, TGF-β induces the expression of the TIM-3 ligand Gal-9 through the Smad3 pathway. This brings inspiration for our next research (29).
Su et al. found that TIM-3 positivity in tumor cells or tumor-infiltrating lymphocytes was an independent prognostic factor of worse recurrence-free survival and OS in patients with surgically resected lung adenocarcinoma (17). As the most common immune cells in the TME, TAMs play an important role in the development of lung cancer (30). However, the association between TIM-3 expression in TAMs and its prognostic significance in NSCLC has not yet been elucidated. In this study, we found that patients with different levels of TIM-3 expression in TAMs or CD68+/CD163+ TAM density combinations had different prognoses. These findings stratified patients into different subgroups, which is helpful for evaluating the prognosis of patients with NSCLC and adenocarcinoma. In addition, high TIM-3 expression in TAMs in the tumor stroma was significantly correlated with shorter OS in NSCLC and adenocarcinoma. This can be explained by the finding that TIM-3 promotes the tumor-promoting M2 macrophage polarization (31).
Conclusions
Our results demonstrate that high TIM-3 expression in TAMs is an independent predictor of worse prognosis in patients. TIM-3 expression in tumor-associated macrophages may be a promising therapeutic target for NSCLC or adenocarcinoma.
There were some limitations to our study. First, it was a retrospective study and thus subject to bias. Second, we did not use the immunofluorescence technique to analyze TIM-3, CD68, and CD163 co-expression in TAMs in lung cancer tissues. We hope to clarify the mechanism by which TIM-3 expression in TAMS affects the prognosis of patients with NSCLC in the future.
Acknowledgments
Funding: This research was supported by grants from Zhejiang Health Science and Technology Project (Nos. 2019ZD060, 2022KY1365 and 2021C31045).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-227/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-227/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-227/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-227/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Zhoushan Hospital (No. 2018-146). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang S, Sun K, Zheng R, et al. Cancer incidence and mortality in China. Journal of the National Cancer Center 2021;1:2-11. [Crossref]
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Lu D, Ma Z, Huang D, et al. Clinicopathological characteristics and prognostic significance of HDAC11 protein expression in non-small cell lung cancer: a retrospective study. Transl Lung Cancer Res 2022;11:1119-31. [Crossref] [PubMed]
- Li Z, Fan L, Wu Y, et al. Analysis of the prognostic role and biological characteristics of circulating tumor cell-associated white blood cell clusters in non-small cell lung cancer. J Thorac Dis 2022;14:1544-55. [Crossref] [PubMed]
- Giatromanolaki A, Kouroupi M, Balaska K, et al. A Novel Lipofuscin-detecting Marker of Senescence Relates With Hypoxia, Dysregulated Autophagy and With Poor Prognosis in Non-small-cell-lung Cancer. In Vivo 2020;34:3187-93. [Crossref] [PubMed]
- Dosaka-Akita H, Hommura F, Mishina T, et al. A risk-stratification model of non-small cell lung cancers using cyclin E, Ki-67, and ras p21: different roles of G1 cyclins in cell proliferation and prognosis. Cancer Res 2001;61:2500-4. [PubMed]
- Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett 2015;368:7-13. [Crossref] [PubMed]
- Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy 2017;9:289-302. [Crossref] [PubMed]
- Mei J, Xiao Z, Guo C, et al. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget 2016;7:34217-28. [Crossref] [PubMed]
- Freeman GJ, Casasnovas JM, Umetsu DT, et al. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 2010;235:172-89. [Crossref] [PubMed]
- Baghdadi M, Jinushi M. The impact of the TIM gene family on tumor immunity and immunosuppression. Cell Mol Immunol 2014;11:41-8. [Crossref] [PubMed]
- Sabins NC, Chornoguz O, Leander K, et al. TIM-3 Engagement Promotes Effector Memory T Cell Differentiation of Human Antigen-Specific CD8 T Cells by Activating mTORC1. J Immunol 2017;199:4091-102. [Crossref] [PubMed]
- Gleason MK, Lenvik TR, McCullar V, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood 2012;119:3064-72. [Crossref] [PubMed]
- Schwartz JA, Clayton KL, Mujib S, et al. Tim-3 is a Marker of Plasmacytoid Dendritic Cell Dysfunction during HIV Infection and Is Associated with the Recruitment of IRF7 and p85 into Lysosomes and with the Submembrane Displacement of TLR9. J Immunol 2017;198:3181-94. [Crossref] [PubMed]
- Ocaña-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I. TIM-3 Regulates Distinct Functions in Macrophages. Front Immunol 2016;7:229. [Crossref] [PubMed]
- Gorman JV, Colgan JD. Regulation of T cell responses by the receptor molecule Tim-3. Immunol Res 2014;59:56-65. [Crossref] [PubMed]
- Su H, Xie H, Dai C, et al. Characterization of TIM-3 expression and its prognostic value in patients with surgically resected lung adenocarcinoma. Lung Cancer 2018;121:18-24. [Crossref] [PubMed]
- Yan W, Liu X, Ma H, et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 2015;64:1593-604. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Jia K, He Y, Dziadziuszko R, et al. T cell immunoglobulin and mucin-domaincontaining-3 in non-small cell lung cancer. Transl Lung Cancer Res 2019;8:895-906. [Crossref] [PubMed]
- Dora D, Rivard C, Yu H, et al. Characterization of Tumor-Associated Macrophages and the Immune Microenvironment in Limited-Stage Neuroendocrine-High and -Low Small Cell Lung Cancer. Biology (Basel) 2021;10:502. [Crossref] [PubMed]
- Dora D, Rivard C, Yu H, et al. Protein Expression of immune checkpoints STING and MHCII in small cell lung cancer. Cancer Immunol Immunother 2023;72:561-78. [Crossref] [PubMed]
- Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002;415:536-41. [Crossref] [PubMed]
- Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer 2020;8:e000911. [Crossref] [PubMed]
- Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol 2018;11:126. [Crossref] [PubMed]
- Yang M, Li J, Gu P, et al. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater 2021;6:1973-87. [Crossref] [PubMed]
- Fucikova J, Rakova J, Hensler M, et al. TIM-3 Dictates Functional Orientation of the Immune Infiltrate in Ovarian Cancer. Clin Cancer Res 2019;25:4820-31. [Crossref] [PubMed]
- Li H, Wu K, Tao K, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012;56:1342-51. [Crossref] [PubMed]
- Selnø ATH, Schlichtner S, Yasinska IM, et al. Transforming growth factor beta type 1 (TGF-β) and hypoxia-inducible factor 1 (HIF-1) transcription complex as master regulators of the immunosuppressive protein galectin-9 expression in human cancer and embryonic cells. Aging (Albany NY) 2020;12:23478-96. [Crossref] [PubMed]
- Sarode P, Schaefer MB, Grimminger F, et al. Macrophage and Tumor Cell Cross-Talk Is Fundamental for Lung Tumor Progression: We Need to Talk. Front Oncol 2020;10:324. [Crossref] [PubMed]
- Jiang X, Zhou T, Xiao Y, et al. Tim-3 promotes tumor-promoting M2 macrophage polarization by binding to STAT1 and suppressing the STAT1-miR-155 signaling axis. Oncoimmunology 2016;5:e1211219. [Crossref] [PubMed]
(English Language Editor: J. Jones)


