
Development and validation of a novel M1 macrophage-related gene prognostic signature for lung cancer
Highlight box
Key findings
• M1 macrophage-related subtypes of LC were identified and a seven M1 macrophage-related gene signature could predict prognosis for LC patients.
What is known and what is new?
• M1 macrophages are essential for tumor growth, invasion and metastasis in cancer.
• We first present M1 macrophage-related subtypes of LC and M1 macrophage-related biomarkers.
What is the implication, and what should change now?
• M1 macrophage-related biomarkers are strongly correlated with the prognosis of LC patients.
Introduction
Lung cancer (LC) is the most common cancer; it has the highest incidence and mortality rates. Adenocarcinoma is the most common type of LC (1). Although many efforts have been made to treat and diagnose LC, the 5-year survival rate of LC is only approximately 20% (2). Therefore, it is critical to discover an ideal novel target for the early-stage detection, prognosis and therapeutic intervention of LC.
Although infiltrating immune cells can control the progression of tumors, tumors can weaken the function of immune cells to protect themselves by generating a tumor microenvironment (3,4). Macrophages, which can be divided into classic M1 or alternative M2 types in tumor microenvironments, can both promote and suppress cancer growth (5-7). M1 macrophages are considered antitumor factors, while M2 macrophages are considered tumor-promoting factors (8). The presence of M1 macrophages is associated with strong CD8+ tissue-resident memory (TRM) T-cell tumor infiltration and better survival outcomes in patients with LC (9), indicating that this feature is highly clinically relevant. Therefore, M1 macrophages are essential for tumor growth, invasion and metastasis in cancer. However, M1 macrophages have been studied to a lesser extent in LC. Thus, it is important to identify M1 macrophage biomarkers that are beneficial in LC.
Recently, the prognostic gene signatures of autophagy, ferroptosis and immune were established in lung adenocarcinoma (10-12), however, the role of macrophages-related genes in the prognosis of LC remains unclear. Considering M1 macrophages are considered antitumor factors, we focused on the essential role of M1 macrophages in LC to construct an M1-related gene signature for the LC cohort, demonstrating that it has independent prognostic value, as well as exploring the clinical utility for LC patients.
Herein, a systematic study was performed to identify M1 macrophage-related genes in LC using The Cancer Genome Atlas (TCGA) database and to investigate the underlying molecular mechanisms of these genes in LC patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-80/rc).
Methods
Datasets of LC patients
The raw data and clinical details were obtained from the TCGA and the Gene Expression Omnibus (GEO) databases. The cohort contained 507 LC patients for a training set, with the complete clinical information of 507 patients for further analysis from the TCGA database (https://portal.gdc.cancer.gov/repository). The raw data and the clinical information of 442 LC patients for a validation set were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). All the patients did not receive preoperative chemotherapy or radiation and follow-up information was available at least two years. The frozen sections of the samples considered for inclusion in this study. The “rtracklayer” and “dplyr” R packages were used to change gene names from Ensemble IDs to gene symbols through the Ensemble database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Identification and functional annotation of M1 macrophage-related genes in LC
M1 macrophage-related genes were obtained from LC patients in the TCGA database, M1 macrophage is calculated based on the CIBERSORT, and the top 10 genes were selected to display based on the P value. Comprehensive networks were used to explore the relationship between these macrophage-related genes and M1 macrophages. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed by applying the “ClusterProfiler” R package. The statistical significance of GO and KEGG analysis used the q<0.05.
Construction and validation of a prognostic gene model in the TCGA-LC cohort
To explore the prognostic value of M1 macrophage-related genes in LC patients, 11 prognostic M1 macrophage-related genes were identified using univariate Cox analysis. Then, a prognostic model of 11 prognostic M1 macrophage-related genes was developed by least absolute shrinkage and selection operator (LASSO) Cox regression through the “glmnet” R package. Nonzero coefficients were used as variables to construct a prognostic model when the lambda condition was minimal. The risk score was calculated as follows: risk score = sum (expression level of each gene × corresponding coefficient). Then, TCGA-LC patients were divided into high- or low-risk groups by the median risk score. Kaplan-Meier curves were drawn and analyzed by the R package “survival”. Calculation of hazard ratios (HRs) with 95% confidence intervals (CIs) was based on Cox proportional hazards analysis. The seven-gene signature was assessed to predict LC patient prognosis from the GEO cohort. The P value, risk coefficient (HR) and CI were analyzed by univariate and multivariate survival analyses.
Comparative analysis of immune infiltration between LC subtypes
The immune infiltration of the two subtypes was estimated by CIBERSORT algorithms using the “immunedeconv” R package and visualized as heatmaps and boxplots. In addition, 8 immune checkpoint-related genes were selected, including ATIC, OLA1, CTLA4, PDCD1, CD274, IDO1, HAVCR2 and PDCD1LG2, and the correlation of these eight genes was visualized. Statistical significance was determined by the Wilcox test, and P<0.05 was considered significant.
Gene set enrichment analysis (GSEA)
The local version of GSEA (http://software.broadinstitute.org/gsea/index.jsp) was used to investigate the biological function of the two LC subtypes. The GO biological processes (BPs) and KEGG pathways were utilized for GSEA independently. The false defection rate q<0.05 was used to select the BPs and KEGG pathways enriched in each subtype.
Statistical analysis
All statistical analyses were conducted in the R language (Version 3.6.3). Univariate and multivariate survival analysis were developed to identify independent prognostic factors in LC using the criteria of P<0.05. Kaplan-Meier survival analysis was conducted between high- and low-risk groups and evaluated using a log-rank test. A two-sided P value <0.05 was considered statistically significant. GSEA analysis was used to select the BPs and KEGG pathways using the criteria of q<0.05.
Results
Identification of M1 macrophage-related genes in LC
To explore the distribution characteristics of M1 macrophages in LC patients, we first explored M1 macrophage-related genes in LC patients in the TCGA database. In total, 87 M1 macrophage-related genes were identified (available online: https://cdn.amegroups.cn/static/public/jtd-23-80-1.xlsx), and the top 10 genes positively correlated with M1 macrophages are shown in Figure 1A (including CXCL10, CXCL9, CXCL11, GBP1, CALHM6, GBP4, GBP5, WARS1, STAT1 and IFNG).
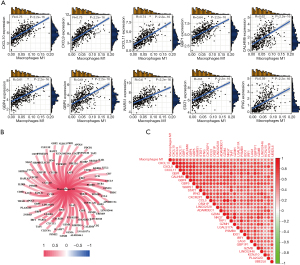
Next, comprehensive networks were proposed to explore the relationship between these macrophage-related genes and M1 macrophages. Interestingly, all these genes were positively associated with M1 macrophages, and the top 30 genes were also positively correlated with each other (Figure 1B,1C).
Functional annotation of M1 macrophage-related genes in LC
We further investigated the underlying molecular mechanisms of these M1 macrophage-related genes, and functional enrichment analyses, including GO and KEGG analyses, were performed. The enriched BPs of GO were mainly associated with T-cell activation, the cytokine-mediated signaling pathway, mononuclear cell differentiation, the chemokine-mediated signaling pathway and the response to interferon-gamma (IFN-γ) (Figure 2A). The enriched molecular functions (MFs) and cell components (CCs) of GO were similar to the those of the BPs (Figure 2A and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-2.xlsx). Comprehensive networks were proposed to explore the relationship between the macrophage-related genes and BPs of GO (including T-cell activation, cellular response to IFN, response to IFN, T-cell differentiation and regulation of T-cell activation) in Figure 2B. The genes IFNG, CCL5, IRF1, LAG and PLA2G2D were related to three BPs (Figure 2B).
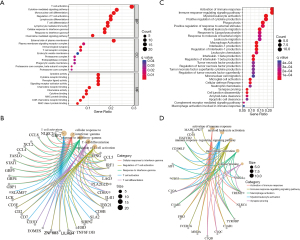
Furthermore, KEGG analysis found that the activation of the immune response, myeloid leukocyte activation, leukocyte migration, phagocytosis and the positive regulation of cytokine production (Figure 2C and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-2.xlsx) were enriched, which further supports the GO results. Comprehensive networks were used to further explore the relationship between these macrophage-related genes and KEGG pathways. We found that five genes, VSIG4, TREM2, TYROBP, C1QA and HAVCR2, were related to three KEGG pathways (Figure 2D).
These results suggest that M1 macrophage-related genes might play an important role in the activation of the immune response and cytokine-mediated signaling pathway in the occurrence and development of LC.
Identification of seven M1 macrophage-related genes in LC
To explore the prognostic value of M1 macrophage-related genes in a total of 507 TCGA-LC patients, 11 prognostic M1 macrophage-related genes were identified using univariate Cox analysis, including STAT1, TAP1, UBE2L6, TAP2, CXCR6, GZMK, PSMB8, SH2D1A, CD3D, CD2 and LCK (Figure 3A).
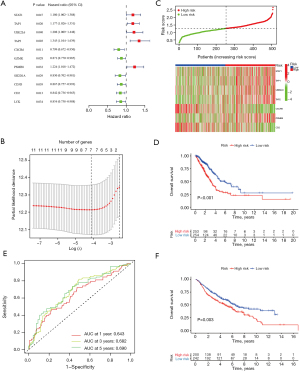
We further used LASSO Cox regression analysis to prioritize these candidate macrophage-related genes, and a seven-gene signature was determined. The risk score of each patient was generated using the following risk score formula: risk score = (0.0458) × STAT1 + (0.0474) × TAP1 + (0.0633) × UBE2L6 + (0.3768) × TAP2 + (−0.3185) × CXCR6 + (0.0319) × PSMB8 + (−0.1375) × CD2 (Figure 3B,3C and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-3.xlsx). Given the relationship between these seven genes and the LC subtypes, LC patients were divided into a high-risk group and a low-risk group (Figure 3C). Kaplan-Meier (KM) curves of these seven M1 macrophage-related genes further showed that the low-risk group had a longer overall survival (OS) time than the high-risk group (P<0.001; Figure 3D). Receiver operating characteristic (ROC) curves were used to further assess the prognostic values of these seven genes. The 1-, 3- and 5-year areas under the ROC curves (AUCs) for OS were 0.643, 0.682, and 0.690, respectively (Figure 3E). Additionally, the prognostic value of this seven-gene signature could be validated using a total of 442 LC patients from the GEO cohort (Figure 3F and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-3.xlsx). The KM curves of these genes between the low- and high-risk groups were consistent with the results of the TCGA cohort (Figure 3F and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-3.xlsx). These results suggest that these seven macrophage-related genes might act as key regulators in the development of LC.
Validation of the subtype classification of the seven macrophage-related gene signature
The above high- and low-risk groups were associated with clinical outcomes. We further assessed whether the subtype classification of the seven macrophage-related gene signature could serve as an independent prognostic factor using univariate and multivariate survival analyses. The Cox proportional hazards regression model was constructed in the TCGA-LC cohort through candidate independent variables (age, sex, stage, and risk score of the model). Univariate survival analysis found that the subtype of the seven macrophage-related gene signature was significantly associated with an adverse prognosis in patients with LC (HR 3.810, 95% CI: 2.584–5.618, P<0.001, Figure 4A and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-4.xlsx). Moreover, multivariate survival analysis revealed that the subtype was an independent prognostic factor independent of age, sex and tumor stage (HR 2.923, 95% CI: 1.951–4.381, P<0.001, Figure 4B and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-4.xlsx).
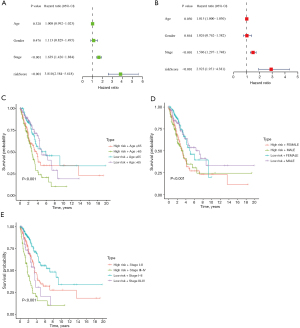
Next, we also found that the high- and low-risk groups were positively correlated with several LC classifications, including age (P<0.001), sex (P<0.001) and tumor stage (P<0.001) (Figure 4C-4E). These results suggest that the above subtype classification was an effective independent prognostic factor in LC.
Correlation of the two subtypes with immune infiltration in the TCGA-LC cohort
Given the two identified subtypes determined by the seven M1 macrophage-related genes, we further explored whether these subtypes were related to immune infiltration. Compared with the low-risk group, the high-risk group showed significant differences in the infiltration of immune cells, such as plasma cells, follicular helper T cells, resting natural killer (NK) cells, M0 macrophages, M1 macrophages, M2 macrophages and activated dendritic cells (Figure 5A). In addition, we also noted that the seven M1 macrophage-related genes were significantly associated with the infiltration of immune cells (Figure 5B). The model of the two identified subtypes was correlated with checkpoint inhibitors, including ATIC, OLA1, CTLA4, PDCD1, CD274 and HAVCR2 (Figure 5C). These results indicate that the two subtypes were correlated with immune infiltration in the TCGA-LC cohort.

Signaling pathways in two subtypes in the TCGA-LC cohort
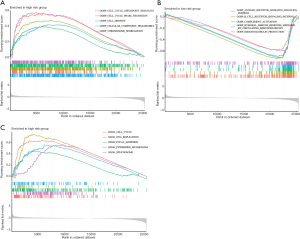
Given that the seven M1 macrophage-related genes were correlated with survival time, we further investigated the hallmark pathways related to the low-risk group and high-risk group using GSEA. There were 5 upregulated tumor-related BPs identified in the high-risk group, including cell cycle checkpoint signaling, cell cycle phase transition, cell growth, cellular component disassembly and chromosome segregation (Figure 6A and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-5.xlsx). In contrast, there were 5 upregulated immune-related BPs identified in the low-risk group, including the antigen receptor-mediated signaling pathway, cell receptor signaling pathway, complement activation, humoral immune response mediated by circulating immunoglobulin and immunoglobulin production (Figure 6B and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-5.xlsx). Consistent with the results of BP analysis, there were 5 upregulated KEGG pathways identified in the high-risk group, including cell cycle, DNA replication, focal adhesion, pyrimidine metabolism and spliceosome (Figure 6C and available online: https://cdn.amegroups.cn/static/public/jtd-23-80-5.xlsx). These results suggest that the pathways of tumor cell proliferation might act as key regulators in the development of LC in the high-risk group.
Discussion
In this study, we first identified M1 macrophage-related genes using TCGA data, and these genes might play an important role in the activation of the immune response and cytokine-mediated signaling pathway in LC. A seven M1 macrophage-related gene signature (including STAT1, TAP1, UBE2L6, TAP2, CXCR6, PSMB8 and CD2) was identified in LC using LASSO Cox regression analysis. Two subtypes (low risk and high risk) of LC patients were constructed based on the seven M1 macrophage-related gene signature. Univariate and multivariate survival analyses confirmed that subtype classification was an effective independent prognostic factor. In addition, the two subtypes correlated with immune infiltration, and GSEA revealed that the pathways of tumor cell proliferation and immune-related BPs might play an important role in LC in the high-risk group and low-risk group, respectively. In summary, M1 macrophage-related biomarkers were strongly correlated with the prognosis of LC patients.
Classic M1 macrophages are regarded as proinflammatory and antitumor factors, whereas alternative M2 macrophages are regarded as anti-inflammatory and tumor-promoting factors (8,13). Moreover, M1 macrophages are related to strong CD8+ T-cell tumor infiltration and better survival outcomes in LC (9). In this study, 87 M1 macrophage-related genes were associated with activation of the immune response and cytokine-mediated signaling pathway in LC, which is consistent with previous studies (8,9,13). Furthermore, 87 M1 macrophage-related genes were identified in this study.
Due to the correlation between the status of M1 macrophages and clinical outcomes in cancers (8,9,13), a gene signature was constructed to divide LC patients into high- and low-risk groups. Based on lasso regression analysis, we identified that a seven-gene signature, including STAT1, TAP1, UBE2L6, TAP2, CXCR6, PSMB8 and CD2, showed a substantial effect on survival prediction. Moreover, the two subtypes correlated with immune infiltration in the TCGA-LC cohort. GSEA revealed that the pathways of tumor cell proliferation and immune-related BPs might act as key regulators in the development of LC in the high-risk group and low-risk group, respectively.
Some of these seven genes have played important roles in LC. Signal transducer and activator of transcription 1 (STAT1) regulates cellular responses to IFNs, cytokines and growth factors (14). Moreover, it was reported that KHSRP promoted cell invasion and metastasis by activating the STAT1 signaling pathway in LC (15). TAP1, encoding antigen peptide transporter 1, mediates the translocation of peptide antigens from the cytosol to the endoplasmic reticulum (ER) (16). The expression of TAP1 is related to survival outcomes in breast, lung, liver, and ovarian cancer (17). Ubiquitin/ISG15-conjugating enzyme (UBE2L6) induces the ubiquitination of the tumor suppressor p53/TP53 (18). CXCR6 deficiency impaired therapy resistance and CD8+ T-cell recruitment in LC (19). In addition, knockdown of KNTC1 influenced non-small cell LC by regulating PSMB8 (20). T-cell surface antigen CD2 mediates adhesion between T cells and others (21).
Some studies related to prognostic gene signature, have been reported in LC (19-21). Li et al. constructed a combined ferroptosis and immune prognostic signature for lung adenocarcinoma (10). Gong et al. developed a novel signature based on autophagy-related lncRNA in lung adenocarcinoma (11). Peng et al. constructed a prognostic risk signature based on six ferroptosis, necroptosis, and pyroptosis-related lncRNAs in LC (12). However, the role of macrophages-related genes in the prognosis of LC remains unclear. In this study, M1 macrophage-related subtypes of LC were identified and were closely associated with immune infiltration. The gene signature involved in M1 macrophage-related genes could help make a distinction and predict prognosis for LC patients.
This study has some limitations. The roles of seven genes in LC should be further explored in vitro and in vivo. In addition, the mechanism of these genes in LC progression also should be explored, which may give new strategies for LC treatment.
Taken together, our findings indicate that the M1 macrophage status was closely related to the activation of the immune response and cytokine-mediated signaling pathway in LC patients. Gene signatures related to M1 macrophages further predicted the prognosis of LC patients. For precision medicine, this feature can satisfy clinical needs and propose potential therapeutic regimens for LC management to a certain extent.
Conclusions
M1 macrophage-related subtypes of LC were identified and closely associated with immune infiltration. The gene signature involved in M1 macrophage-related genes could help make a distinction and predict prognosis for LC patients.
Acknowledgments
Funding: This work was supported by the Science and Technology Research Programme of the Health Commission of Hebei Province (No. 20201185).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-80/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-80/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-80/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen C, Guo Q, Song Y, et al. SKA1/2/3 serves as a biomarker for poor prognosis in human lung adenocarcinoma. Transl Lung Cancer Res 2020;9:218-31. [Crossref] [PubMed]
- Sun L, Han T, Zhang X, et al. PRRX1 isoform PRRX1A regulates the stemness phenotype and epithelial-mesenchymal transition (EMT) of cancer stem-like cells (CSCs) derived from non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 2020;9:731-44. [Crossref] [PubMed]
- Luo Y, Barrios-Rodiles M, Gupta GD, et al. Atypical function of a centrosomal module in WNT signalling drives contextual cancer cell motility. Nat Commun 2019;10:2356. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Pellizzari G, Hoskin C, Crescioli S, et al. IgE re-programs alternatively-activated human macrophages towards pro-inflammatory anti-tumoural states. EBioMedicine 2019;43:67-81. [Crossref] [PubMed]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66:605-12. [Crossref] [PubMed]
- Szebeni GJ, Vizler C, Kitajka K, et al. Inflammation and Cancer: Extra- and Intracellular Determinants of Tumor-Associated Macrophages as Tumor Promoters. Mediators Inflamm 2017;2017:9294018. [Crossref] [PubMed]
- Yunna C, Mengru H, Lei W, et al. Macrophage M1/M2 polarization. Eur J Pharmacol 2020;877:173090. [Crossref] [PubMed]
- Garrido-Martin EM, Mellows TWP, Clarke J, et al. M1(hot) tumor-associated macrophages boost tissue-resident memory T cells infiltration and survival in human lung cancer. J Immunother Cancer 2020;8:e000778. [Crossref] [PubMed]
- Li H, Ge Y, Fei G, et al. Development and validation of a combined ferroptosis and immune prognostic signature for lung adenocarcinoma. Transl Cancer Res 2022;11:3620-33. [Crossref] [PubMed]
- Gong Z, Li Q, Li J, et al. A novel signature based on autophagy-related lncRNA for prognostic prediction and candidate drugs for lung adenocarcinoma. Transl Cancer Res 2022;11:14-28. [Crossref] [PubMed]
- Peng L, Ji J, Zhang C, et al. Development and validation of a prognostic risk signature for lung adenocarcinoma constructed by six ferroptosis, necroptosis, and pyroptosis-related lncRNAs. J Thorac Dis 2022;14:3955-74. [Crossref] [PubMed]
- Geraghty T, Rajagopalan A, Aslam R, et al. Positive Allosteric Modulation of CD11b as a Novel Therapeutic Strategy Against Lung Cancer. Front Oncol 2020;10:748. [Crossref] [PubMed]
- Li M, Liu Y, Fu Y, et al. Interleukin-35 inhibits lipopolysaccharide-induced endothelial cell activation by downregulating inflammation and apoptosis. Exp Cell Res 2021;407:112784. [Crossref] [PubMed]
- Zhang Y, Liu Z, Yang X, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics 2021;11:1473-92. [Crossref] [PubMed]
- Fischbach H, Döring M, Nikles D, et al. Ultrasensitive quantification of TAP-dependent antigen compartmentalization in scarce primary immune cell subsets. Nat Commun 2015;6:6199. [Crossref] [PubMed]
- Tabassum A, Samdani MN, Dhali TC, et al. Transporter associated with antigen processing 1 (TAP1) expression and prognostic analysis in breast, lung, liver, and ovarian cancer. J Mol Med (Berl) 2021;99:1293-309. [Crossref] [PubMed]
- Park JH, Yang SW, Park JM, et al. Positive feedback regulation of p53 transactivity by DNA damage-induced ISG15 modification. Nat Commun 2016;7:12513. [Crossref] [PubMed]
- Karaki S, Blanc C, Tran T, et al. CXCR6 deficiency impairs cancer vaccine efficacy and CD8(+) resident memory T-cell recruitment in head and neck and lung tumors. J Immunother Cancer 2021;9:e001948. [Crossref] [PubMed]
- Liu R, Liu R, Guo Z, et al. shRNA-mediated knockdown of KNTC1 inhibits non-small-cell lung cancer through regulating PSMB8. Cell Death Dis 2022;13:685. [Crossref] [PubMed]
- Li B, Lu Y, Zhong MC, et al. Cis interactions between CD2 and its ligands on T cells are required for T cell activation. Sci Immunol 2022;7:eabn6373. [Crossref] [PubMed]


