
A uniport subxiphoid approach with a modified sternum retractor is safe and feasible for anterior mediastinal tumors
Highlight box
Key findings
• Uniport subxiphoid mediastinal surgery with our modified sternum retractor is a feasible and safe procedure, especially for large tumors.
What is known and what is new?
• Subxiphoid surgery has been reported to be associated with less pain and more feasibility than lateral surgery in the removal of large tumors. Scholars have invented various sternum retractors to facilitate the surgery. However, to date, there have been few reports on the uniport approach.
• With the help of our modified sternum retractor, the feasibility and benefits of the uniport subxiphoid approach may exceed those of unilateral surgery.
What is the implication, and what should change now?
• This surgical approach can be applied to early stage and select locally advanced tumors, especially large tumors.
Introduction
Anterior mediastinal tumors, including various types of neoplasms, are relatively rare. Median sternotomy, which provides excellent exposure to the anterior mediastinum, has been the standard surgical approach for tumors in this area for decades. After splitting the sternum, the entire tumor along with the structures involved can be removed, guaranteeing radical resection. However, in addition to the tremendous surgical trauma, this incision also leaves a scar as long as 20–30 cm. In the last decade, there has been a rapid increase in the use of minimally invasive procedures (MIPs) to treat tumors located in the anterior mediastinum (1-4). For early stage tumors, compared to median sternotomy, MIPs yield similar oncological results while also minimizing the surgical trauma, improving the exposure of surgical area, reducing postoperative pain, and accelerating recovery. MIPs are not routinely recommended, but are considered under the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of early stage diseases at specialized centers by experienced surgeons (5).
MIPs, including video-assisted thoracoscopic surgery (VATS), robotic surgery, and other minimally invasive surgical procedures, are usually performed using cervical, lateral thoracic, or subxiphoid approaches. The subxiphoid approach has been reported to be associated with less pain and have more feasibility than lateral surgery in the removal of large tumors (6-8); however, some difficulties may arise in accessing the anterior mediastinum from below. Scholars have invented various sternum retractors to facilitate the surgery (7-9). We describe the experience of a single team in terms of the feasibility and benefits of uniport subxiphoid surgery with a modified sternum retractor compared to unilateral surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-244/rc).
Methods
Eligibility
Patients with mediastinal tumors located in the anterior mediastinum who underwent VATS using the uniport subxiphoid or unilateral approach at the Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai Jiao Tong University between September 2018 to December 2021 were retrospectively enrolled in this study. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had pleural dissemination; (II) had a recurrent tumor; (III) had to undergo conversion to open procedure due to tumor invasion; and/or (IV) had to undergo concurrent surgery for other chest tumors. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1970). Informed consent was waived as this was a retrospective study using only de-identified data.
Surgical procedure
In the uniport subxiphoid VATS (USVATS) group, each patient was placed in the supine position on the operating table with their legs open. The surgeon stood between the patient’s legs, and the assistant stood on the right side of the patient. Under general anesthesia, a single- or double-lumen endotracheal intubation was performed to conduct lung ventilation during the procedure. A 5-cm vertical incision approximately 1-cm caudal to the xiphoid process was usually made followed by the installment of a modified sternum retractor, which could raise the sternum by 6–8 cm to facilitate access to the anterior mediastinum from below. The modified retractor consists of a traction frame and hooks of various sizes that can be replaced according to different needs (Figure 1A). After being placed at the subxiphoid position, the hook is connected to a traction frame (Figure 1B). A sealed incision protector (GM-QP-S-60A-220, V-strong, Jiangsu Grit Medical Technology Co., China) was introduced in the procedure to establish Carbon dioxide (CO2) insufflation as needed (seen in Figure 1C).

In the unilateral VATS (LVATS) group, the patient’s surgical side was raised by 30–45 degrees. Under general anesthesia, a double-lumen endotracheal intubation was performed to provide selective lung ventilation during the procedure. Usually, three 1-cm incisions were made, of which, 2 incisions were made in the 2nd or 3rd and 5th intercostal anterior axillary line, and the 3rd was made in the 5th intercostal midclavicular line. A thoracoscope was placed in the port located at the 5th intercostal space at the anterior axillary line. In some instances, an additional subxiphoid incision was made to remove a large tumor. CO2 insufflation was usually necessary to create a larger space at the retrosternum area.
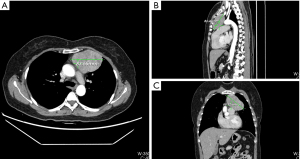
In both groups, a total thymectomy was routinely performed with the tumor resection with safe surgical margins as per the International Thymic Malignancy Interest Group criteria (10). During the operation, the no-touch technique was employed, and the tumor was not clamped or squeezed, as doing so can damage the tumor and increase the risk of pleural dissemination. Rather than removing the tumor separately from the thymus, an en-bloc resection, which included the tumor and thymus, was performed. Once the specimen was completely removed, a protective retrieval bag was used to remove the specimen. Patients in different surgical group were in the same analgesic managements. Figure 1 showed our retractor in detail and the well-exposed operative field. Figure 2 showed one of the largest tumors in USVATS, as large as 10 cm.
Data collection and statistical analysis
Patient characteristics, tumor characteristics (i.e., pathology, invasion, and tumor size) and perioperative findings [i.e., blood loss, operation time, conversion, complications, drainage duration, length of postoperative stay, pain score, use of additional non-steroidal anti-inflammatory drugs (NSAIDs), and surgical procedure] were prospectively recorded. Postoperative pain was evaluated using the visual analogue scale (VAS), under which, scores can range from 0 (no pain) to 10 (maximal pain) (11).
The categorical variables were analyzed using the χ2 test or Fisher’s exact test as appropriate. The continuous variables were compared using the Students’ t-test or Wilcoxon rank-sum test. The log-rank test was used to evaluate the differences among the groups. All the statistical analyses were conducted using SPSS Statistics 19.0 software (SPSS Inc.). A 2-sided P value of <0.05 was considered statistically significant.
Results
A total of 44 patients were enrolled in this study, including 16 patients in the USVATS group and 28 patients in the LVATS group. Of the whole cohort, 26 (59.1%) patients were male and 18 (40.9%) were female. The patients had a median age of 56 years (range, 24–79 years). With the exception of tumor size (USVATS 7.9±1.6 cm vs. LVATS 5.1±2.4 cm, P<0.001), the baseline data of the patients in the 2 groups, including their age, hypertension and diabetes mellitus status, and body mass index (BMI), were comparable. The final pathology results and tumor invasion rates were also similar between the 2 groups (Table 1). One patient with thymic carcinoma in USVATS received induction chemotherapy followed by surgery. His tumor invaded the right middle lobe. Thus, a wedge resection of involved lung tissue was performed with no conversion or postoperative complication occurred in this case.
Table 1
| Characteristics | Total (n=44) | USVATS (n=16) | LVATS (n=28) | P value |
|---|---|---|---|---|
| Age, year, median [range] | 56 [24–79] | 57 [33–79] | 56 [24–70] | 0.525 |
| Sex, male, n (%) | 26 (59.1) | 8 (50.0) | 18 (64.3) | 0.354 |
| BMI >24 kg/m2, yes, n (%) | 22 (50.0) | 7 (43.8) | 15 (53.6) | 0.531 |
| Hypertension, yes, n (%) | 12 (27.3) | 4 (25.0) | 8 (28.6) | 1.000 |
| Diabetes mellitus, yes, n (%) | 6 (13.7) | 2 (12.5) | 4 (14.3) | 1.000 |
| Pathology, n (%) | 0.689 | |||
| TET | 36 (81.8) | 14 (87.5) | 22 (78.6) | |
| Germ-cell tumor | 4 (9.1) | 1 (6.3) | 3 (10.7) | |
| Lymphoma | 4 (9.1) | 1 (6.3) | 3 (10.7) | |
| Tumor size, cm, mean ± SD | 6.1±2.5 | 7.9±1.6 | 5.1±2.4 | <0.001 |
| Tumor invasion*, yes, n (%) | 5 (11.4) | 3 (18.8) | 2 (7.1) | 0.336 |
*, in USVATS group, 1 patient had lung invasion, 1 patient had left innominate vein invasion, and 1 patient had pericardium invasion. In LVATS group, both of the 2 patients had pericardium invasion. USVATS, uniport subxiphoid video-assisted thoracoscopic surgery; LVATS, unilateral video-assisted thoracoscopic surgery; BMI, body mass index; TET, thymic epithelial tumor; SD, standard deviation.
The operation time was significantly longer in the USVATS group than the LVATS group (115±19 vs. 83±30 min, P<0.001), while the VAS score at 1st postoperative day (1.9±1.1 vs. 3.1±1.1, P<0.001) and the moderate-to-severe pain level (a VAS score >3) (6.3% vs. 32.1%, P=0.049) were better in the USVATS group than the LVATS group. However, the need for additional NSAIDs in the 2 groups was similar (31.3% vs. 42.9%, P=0.447). Blood loss in surgery, the conversion rate, the draining duration, the postoperative stay, and the postoperative complications were similar in both groups. In the USVATS group, there was 1 case of atrial fibrillation. In the LVATS group, there was 1 case of atrial fibrillation and 1 case of pulmonary embolism. No perioperative mortality was observed (Table 2).
Table 2
| Parameters | USVATS (n=16) | LVATS (n=28) | P value |
|---|---|---|---|
| Operation time, minute, mean ± SD | 115±19 | 83±30 | <0.001 |
| Blood loss, >100 mL, n (%) | 0 | 0 | 1.000 |
| Conversion, yes, n (%) | 0 | 0 | 1.000 |
| Draining duration, day, mean ± SD | 2.9±1.0 | 3.0±0.7 | 0.732 |
| Postoperative stay, day, mean ± SD | 3.4±1.4 | 4.2±2.1 | 0.173 |
| Complications*, yes, n (%) | 1 (6.3) | 2 (7.1) | 1.000 |
| VAS at POD 1, mean ± SD | 1.9±1.1 | 3.1±1.1 | <0.001 |
| VAS >3, n (%) | 1 (6.3) | 9 (32.1) | 0.049 |
| Use of non-steroidal anti-inflammatory drugs, yes, n (%) | 5 (31.3) | 12 (42.9) | 0.447 |
*, in the USVATS group, 1 patient had atrial fibrillation. In the LVATS group, 1 patient had atrial fibrillation and 1 patient had pulmonary embolism. There were no cases of perioperative mortality. USVATS, uniport subxiphoid video-assisted thoracoscopic surgery; LVATS, unilateral video-assisted thoracoscopic surgery; VAS, visual analogue scale; SD, standard deviation; POD, post-operative day.
In 8 patients in the LVATS group, an additional subxiphoid incision was made to remove an oversized tumor. The results of a comparison of the USVATS and LVATS + subxiphoid (LVATSS) groups are shown in Table 3. The baseline characteristics of the patients in the 2 groups, including their age, sex, BMI, hypertension and diabetes mellitus status, final pathology, invasion, and tumor size, were comparable. The perioperative outcomes of both groups were also similar. However, compared to LVATSS group, a shorter postoperative stay (3.4±1.4 vs. 5.3±3.2, P=0.058) was observed in the USVATS group, but the difference was borderline, and the VAS score at 1st postoperative day (1.9±1.1 vs. 3.0±0.8, P=0.020) was also significantly lower in USVATS group.
Table 3
| Parameters | USVATS (n=16) | LVATSS (n=8) | P value |
|---|---|---|---|
| Age (years) median [range] | 57 [33–79] | 55 [25–65] | 0.417 |
| Sex, male, n (%) | 8 (50.0) | 7 (87.5) | 0.178 |
| BMI >24 kg/m2, yes, n (%) | 7 (43.8) | 5 (62.5) | 0.667 |
| Hypertension, yes, n (%) | 4 (25.0) | 2 (25.0) | 1.000 |
| Diabetes mellitus, yes, n (%) | 2 (12.5) | 1 (12.5) | 1.000 |
| Pathology, n (%) | 1.000 | ||
| TET | 14 (87.5) | 7 (87.5) | |
| Germ-cell tumor | 1 (6.3) | 1 (12.5) | |
| Lymphoma | 1 (6.3) | 0 | |
| Tumor size, cm, mean ± SD | 7.9±1.6 | 8.1±2.2 | 0.875 |
| Tumor invasion*, yes, n (%) | 3 (18.8) | 2 (25.0) | 1.000 |
| Operation time, minute, mean ± SD | 115±19 | 101±29 | 0.176 |
| Blood loss, >100 mL, n (%) | 0 | 0 | |
| Conversion, yes, n (%) | 0 | 0 | |
| Draining duration, day, mean ± SD | 2.9±1.0 | 3.3±0.5 | 0.339 |
| Postoperative stay, day, mean ± SD | 3.4±1.4 | 5.3±3.2 | 0.058 |
| Complications&, yes, n (%) | 1 (6.3) | 1 (12.5) | 1.000 |
| VAS at POD 1, mean ± SD | 1.9±1.1 | 3.0±0.8 | 0.020 |
| VAS >3, n (%) | 1 (6.3) | 2 (25.0) | 0.249 |
| Use of non-steroidal anti-inflammatory drugs, yes, n (%) | 5 (31.3) | 4 (50.0) | 0.412 |
*, in the USVATS group, 1 patient had lung invasion, 1 patient had left innominate vein invasion, and 1 patient had pericardium invasion. In the LVATSS group, both of the 2 patients had pericardium invasion; &, in the USVATS group, 1 patient had atrial fibrillation. In the LVATSS group, 1 patient had pulmonary embolism. There were no cases of perioperative mortality. USVATS, uniport subxiphoid video-assisted thoracoscopic surgery; LVATSS, unilateral video-assisted thoracoscopic surgery with additional subxiphoid incision; BMI, body mass index; TET, thymic epithelial tumor; VAS, visual analogue scale; SD, standard deviation; POD, post-operative day.
Discussion
In the last decade, MIPs have been widely applied in mediastinal surgery. The NCCN guidelines recommend the use of MIPs to treat early stage mediastinal tumors at specialized centers with experienced surgeons (5). Currently, VATS with the unilateral approach is still being widely applied. However, this approach creates difficulties in identifying the contralateral phrenic nerve, provides an insufficient exposure to the upper poles of the thymus, and may lead to postoperative pain due to intercostal nerve injury (12). A bilateral approach may provide better exposure to the anterior mediastinum, especially in identifying the bilateral phrenic nerve; however, more intercostal incisions are required, which may increase the operative injury and postoperative pain.
In 1999, Akamine reported a case of thymic cystectomy by VATS using the subxiphoid approach (13). Since then, there have been reports of subxiphoid VATS with or without additional cervical or intercostal incisions. In subxiphoid VATS, surgeons are able to view both the phrenic nerves very clearly and reach high into the area above the level of the left innominate vein (LIV). In addition, by avoiding the intercostal nerve injury, the postoperative pain is significantly relieved. Additionally, with the greater exposure of the anterior mediastinal area, all the associated fat tissues, which may contain ectopic thymic tissue, can be removed. Thus, this approach may also have an advantage in terms of the oncological results (14-16). However, difficulties arise in creating a retrosternal space at the start of this approach.
Cooper (17) first used a retractor to facilitate transcervical thymectomy for myasthenia gravis in 1988, and various sternal retractors have since been invented (7-9). Zielinski et al. (9) reported a double elevation of the sternum in 2013. A 1-cm incision was made at the superior sternal fossa, and 2 hooks were placed in the cervical and subxiphoid incisions and connected to a traction frame. Song et al. (8) reported on the use of a double-sternum retractor similar to Zielinski. Their devices help to expose the upper poles of the thymus, and even help in the fat tissue dissection in the neck. Yang et al. (7) reported on the use of a retractor at the 3rd intercostal parasternal position in 2017. Under both approaches, the sternum can be elevated, thereby enlarging the anterior mediastinal space. However, the intercostal parasternal incision may cause injury to the intercostal and internal mammary arteries, which may lead to a conversion to open surgery due to bleeding. Experienced surgeons can avoid this problem by adjusting the position of the incision, but this adjustment may not improve the access due to individual anatomical particularities. Situations like this can be avoided if an intercostal incision is not made. The device in Zielinski’s report was associated with a low risk of bleeding. However, an auxiliary incision in the 6th intercostal space in the anterior axillary line, which was made to place the thoracoscope, increased the risk of injuring the intercostal muscle, artery and nerve, followed by intercostal neuralgia.
Other types of subxiphoid VATS (without a retractor) may also perform well with the help of CO2 insufflation and additional subcostal incisions (15,16). This method can also provide an extended operation field. Additionally, the placement of surgical instruments in the subcostal incisions can reduce the conflict of instruments and simplify the operation. However, the diaphragm may be injured during the placement of the trocar through a subcostal incision. Further, once there is bleeding in the subcostal incision, diaphragm, or even in the abdominal cavity, it can be extremely difficult to stop the bleeding.
We introduced a uniport subxiphoid approach with a modified sternum retractor to lower the intraoperative risk, reduce postoperative pain, and accelerate postoperative rehabilitation. Our device consists of a traction frame and hooks of various sizes that can be replaced according to different needs. After being placed at the subxiphoid position, the hook is connected to a traction frame. The sternum can be raised by 6–8 cm. This approach provides a well-exposed operation field beneath the level of the LIV. However, the structures above the LIV level become difficult to expose. Additionally, the ventilated lung may shift to the anterior space, affecting the operation view and the exposure of the phrenic nerve to a certain extent. Thus, we introduced a sealed incision protector (GM-QP-S-60A-220, V-strong, Jiangsu Grit Medical Technology Co., China), which has 4 trocars with good mobility for the operation of instruments. This kind of incision protector is widely used in general surgery, gynecology, and urology (18-20). It allows multiple devices to operate and plays a sealing role. With the help of this protector, CO2 insufflation can be established in USVATS.
Notably, CO2 insufflation may affect a patient’s blood pressure. An initial pressure of 6–8 cmH2O is usually safe, and the CO2 flow can be adjusted as needed. After that, the area above the LIV level is well exposed, including the upper poles of thymus, inferior thyroid veins, right brachiocephalic artery, trachea, and even the lower poles of the thyroid. For thymic epithelial tumors (TETs), especially those located at a high level, it is important to perform a total thymectomy that includes the bilateral upper poles. With the help of the insufflation, the ventilated lungs with lower mobility should no longer shift into the operation field and should also provide an excellent view for surgeons to identify the bilateral phrenic nerves. Additionally, the use of a 3-dimensioanl (3D) thoracoscope can further expand the surgical view, and compared to a 30-degree thoracoscope, a 3D thoracoscope with only 1 cable can minimize the clashing of surgical instruments.
Compared to the LVATS group, the tumor size of the USVATS group was significantly larger, but it was similar to that of the LVATSS group. In the LVATS group, due to the intercostal space limitation, it was impossible to remove oversized tumor bodies through the intercostals. An auxiliary subxiphoid incision was often needed to remove the oversized tumors. Compared to USVATS, LVATSS undoubtedly increased the surgical injury and postoperative pain of patients. Similar to previous studies (6-8), the patients who underwent USVATS had significantly lower postoperative VAS pain scores than those with LVATS, and significantly fewer patients had mild-to-severe pain (VAS >3) in the USVATS group than the LVATS group.
Enhanced recovery after surgery (ERAS) has been embraced recently; however, one of the most difficult to achieve ERAS outcomes relates to postoperative pain, especially in thoracic surgery (21-23). USVATS may help to achieve ERAS, as USVATS has a potential advantage in pain management during postoperative hospitalization.
The limitations of this study include the biases that are typically caused by a retrospective design, the small sample size and the personal experience of only one surgeon. Additionally, only the perioperative outcomes between USVATS and LVATS were examined in our study, and the long-term oncological outcomes need to be further clarified.
Conclusions
Uniport subxiphoid mediastinal surgery is a feasible and safe procedure, especially for large tumors, and even for selected patients with induction. Our modified sternum retractor is especially helpful in extending the operation space during uniport subxiphoid mediastinal surgery. Compared to lateral thoracic surgery, this approach has the advantages of less injury and lower postoperative pain, which may lead to a faster recovery. However, its long-term outcomes need to be observed.
Acknowledgments
The abstract has been presented at the 30th ESTS Meeting.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-244/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-244/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-244/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-244/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1970). Informed consent was waived as this was a retrospective study using only de-identified data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang H, Gu Z, Ding J, et al. Perioperative outcomes and long-term survival in clinically early-stage thymic malignancies: video-assisted thoracoscopic thymectomy versus open approaches. J Thorac Dis 2016;8:673-9. [Crossref] [PubMed]
- Gu Z, Chen C, Wang Y, et al. Video-assisted thoracoscopic surgery versus open surgery for Stage I thymic epithelial tumours: a propensity score-matched study. Eur J Cardiothorac Surg 2018;54:1037-44. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-Assisted Thoracic Surgery Thymectomy Versus Sternotomy Thymectomy in Patients With Thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Thymomas and Thymic Carcinomas. Version 1. National Comprehensive Cancer Network, 2022. Retrieved 20 February 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf
- Yano M, Moriyama S, Haneda H, et al. The Subxiphoid Approach Leads to Less Invasive Thoracoscopic Thymectomy Than the Lateral Approach. World J Surg 2017;41:763-70. [Crossref] [PubMed]
- Yang X, Wang S, Jiang J, et al. Comparison of the Perioperative Outcomes for Thoracoscopic Thymectomy Between the Subxiphoid Approach and the Lateral Intercostal Approach for Masaoka-Koga I-II Thymoma: A Propensity Score-Matching Analysis. Ann Surg Oncol 2023;30:506-14. [Crossref] [PubMed]
- Song N, Li Q, Aramini B, et al. Double sternal elevation subxiphoid versus uniportal thoracoscopic thymectomy associated with superior clearance for stage I-II thymic epithelial tumors: Subxiphoid thymectomy compared with VATS. Surgery 2022;172:371-8. [Crossref] [PubMed]
- Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e119. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149-58. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Akamine S, Takahashi T, Oka T, et al. Thymic cystectomy through subxyphoid by video-assisted thoracic surgery. Ann Thorac Surg 1999;68:2339-41. [Crossref] [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Jiang L, Chen H, Hou Z, et al. Subxiphoid Versus Unilateral Video-assisted Thoracoscopic Surgery Thymectomy for Thymomas: A Propensity Score Matching Analysis. Ann Thorac Surg 2022;113:1656-62. [Crossref] [PubMed]
- Zhang L, Li M, Jiang F, et al. Subxiphoid versus lateral intercostal approaches thoracoscopic thymectomy for non-myasthenic early-stage thymoma: A propensity score -matched analysis. Int J Surg 2019;67:13-7. [Crossref] [PubMed]
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Kim SJ, Choi BJ, Lee SC. Overview of single-port laparoscopic surgery for colorectal cancers: past, present, and the future. World J Gastroenterol 2014;20:997-1004. [Crossref] [PubMed]
- Jiang L, Tong D, Li Y, et al. Application of Single-Port Laparoscopic Surgery in Myomectomy. Front Oncol 2021;11:722084. [Crossref] [PubMed]
- Dobbs RW, Halgrimson WR, Talamini S, et al. Single-port robotic surgery: the next generation of minimally invasive urology. World J Urol 2020;38:897-905. [Crossref] [PubMed]
- Carli F. Physiologic considerations of Enhanced Recovery After Surgery (ERAS) programs: implications of the stress response. Can J Anaesth 2015;62:110-9. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Brown LM. "Moving right along" after lung resection, but the data suggest "not so fast". J Thorac Cardiovasc Surg 2016;151:715-6. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


