
Analysis of real-world data to investigate evolving treatment sequencing patterns in advanced non-small cell lung cancers and their impact on survival
Highlight box
Key findings
• Our study represents the 1st investigation on the effectiveness of 2nd line chemotherapy for NSCLC in post-ICI settings, and the results suggest the 2nd line chemotherapy is still effective after 1st line ICI-based treatment.
What is known and what is new?
• Previous studies have shown patients receiving ICI in different settings of LOT had similar treatment duration as well as OS. This study implies ICI may enhance the efficacy of subsequent chemotherapy, even after disease progressed on ICI-based therapy.
What is the implication, and what should change now?
• This study represents the 1st comprehensive analysis of treatment sequencing in aNSCLC in real world settings. Results from this study with respect to the impact of sequencing on clinical outcomes and the effectiveness of post-ICI 2nd line chemotherapy are clinically meaningful.
Introduction
We have witnessed tremendous progress in the last several years in the development of novel therapeutics for the treatment of non-small cell lung cancers (NSCLCs), and the new treatment options have fundamentally changed clinical management of this most commonly occurring cancer in the world. Next generations of tyrosine kinase inhibitors (TKIs) in the front-line setting have significantly improved clinical outcomes in patients with advanced NSCLC (aNSCLC) harboring oncogenic mutations (1-6). For tumors without sensitizing oncogenic mutations, platinum doublet chemotherapy was established in the 1990s as the standard 1st line therapy (7,8). In 2015, two immune checkpoint inhibitors (ICIs), nivolumab and pembrolizumab were approved by FDA for the treatment of aNSCLCs progressed on platinum chemotherapy (9-11). Subsequently, several ICI-containing regimens demonstrated superiority to chemotherapy in the 1st line setting in randomized phase 3 trials (12-16). Soon after, these regimens received regulatory approvals and were incorporated into clinical oncology guidelines replacing platinum chemotherapy as the 1st line treatment options for aNSCLCs without known driver mutations. As a result of this rapidly evolving treatment landscape, and due to differences in how new treatment options are adopted by different clinicians (17), treatment sequencing has become increasingly complex. Since optimal sequencing of systemic therapy can have a profound impact to alter disease trajectory and to achieve maximal clinical benefit, it is critical to understand treatment sequencing patterns in clinical practices and their associations with clinical outcomes.
After disease progression following the initial systemic treatment of aNSCLCs without oncogenic mutations, there are several options for the subsequent therapies. For patients without previous ICI exposure, a single agent of anti-programmed death-1 (PD-1) or anti-programmed death-ligand 1 (PD-L1) antibody is a reasonable approach, provided patients do not have contraindications to ICIs. If a patient received an ICI-containing regimen for the initial treatment, the 2nd line options include docetaxel (with or without ramucirumab), pemetrexed and gemcitabine. While these agents have been recommended in clinical guidelines, the pivotal trials demonstrating their activity were conducted before the ICI era when platinum-based regimens were the standard 1st line therapy. Consequently, it is of great clinical importance to assess how effective these agents are as the 2nd line therapy when the standard 1st line treatment has evolved.
Evidence-based medicine is the guiding principle in clinical practice, and one of the main sources of evidence comes from clinical trials. Real world data (RWD) represents a complementary source of clinical evidence as they augment clinical trial results to provide a more complete picture of a medical intervention in daily clinical practice. We have previously utilized RWD to evaluate treatment response in patient populations that are generally underrepresented in clinical (18), and to develop novel biomarkers (19). RWD is particularly useful for studying treatment sequencing patterns in clinical oncology since generally clinical trial is not a feasible option. In this study, we analyzed the medical records of 13,340 NSCLC patients in the Mount Sinai Health System (MSHS) electronic medical record (EMR) database for their treatment history and clinical outcomes. The primary objectives of the study are: (I) to examine sequencing of systemic treatment in aNSCLC, specifically how sequencing patterns evolved as new treatment options became available; (II) to investigate how different sequencing patterns impacted survival; (III) to assess the effectiveness of the 2nd line regimens after ICI-based treatment became the new standard 1st line therapy. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1481/rc).
Methods
Study cohort
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was confirmed and approved by the Program for the Protection of Human Subjects at the Mount Sinai School of Medicine (IRB-17-01245). Since the study was a retrospective analysis of the patient database, the requirement for informed consent of each patient was waived. The NSCLC cohort [2003–2021] was curated from the Mount Sinai Healthcare system, which contains longitudinal data for approximately 3.9 million patients. Demographic, clinical, and outcome variables were obtained by either extracting these predictors from structured data or curating the relevant information from unstructured clinical notes.
We first identified patients with lung cancer based on International Classification of Diseases 9th or 10th revision (ICD-9 and/or ICD-10) diagnosis codes (ICD-9: 162.*; ICD-10: C34.*) and histology status. Patients who were treated with eligible NCSLC cancer drugs were included in this study. Eligible NCSLC cancer drugs were identified from structured data, and manually reviewed based on National Comprehensive Cancer Network (NCCN) guidelines and Medication Enquiry Databases from NCI (https://seer.cancer.gov/oncologytoolbox/canmed/). The final study cohort included NSCLC patients who have received at least one line of systemic therapy for the advanced disease (n=2,106). The median follow-up time is 11.3 months.
Line of therapies (LOTs) and treatment sequencing patterns
The therapeutic classes for NSCLC were categorized as chemotherapy, ICI, or targeted therapy based on NCCN guidelines. A LOT algorithm was constructed to identify, process, and analyze the treatment patterns for NSCLC patients. The pipeline was implemented and customized to NSCLC treatment based on the modules described in previous studies (20,21). For each patient in the cohort, LOT were calculated as follows:
- Index date and the 1st LOT drugs: set the first day with the first eligible drug in the NCCN guidelines for NSCLC as the index date and the start date of the first LOT.
- Line regimen window: the window was implemented to specify the duration after the date of the first eligible drug in any LOT based on the previous studies (20,21). All the eligible drugs within in the window were defined to be part of the treatment regimen of the LOT.
- Line switch: the date of a new drug used outside of the current line regimen window was defined as the start date of the new LOT.
- Optimization of line regimen windows and line switches: a subset of the data was manually evaluated to guide iterations of pre-specified windows and gaps. Steps 2 and 3 were performed recursively to optimize of the line regimen window and line switches until the accuracy of LOT reached of 95%.
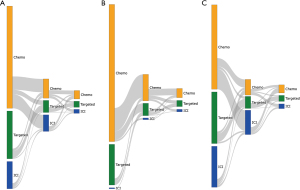
Sankey diagrams were used to depict the overall therapy class distributions and their sequence from L1 to later lines for NSCLC treatment sequencing patterns.
Statistical analysis
Overall survival (OS) and time-to-next treatment (TTNT) were used as clinical endpoints. OS was evaluated from the start of a LOT of interest to the death of a patient recorded in the MSHS death registry. TTNT was calculated by subtracting the start date of the current LOT from the start date of the next LOT. TTNT was used as a surrogate of progression-free survival (PFS) (22). For censored patients, follow-up time was calculated from the start of a LOT to the date of any last activity (i.e., lab test, treatment, etc.).
Cox proportional hazard models were developed for univariate and multivariate analyses. Hazard ratios (HRs) with 95% confidence intervals (CIs) and Wald test-based P values were computed. Kaplan-Meier analyses were used to visualize OS and TTNT for the treatment groups and to compute median time-to-event in each group.
Results
Treatment sequencing patterns in aNSCLC
The selection of the study cohort is illustrated in Figure S1. In the overall study population who had systemic treatment including at least one drug listed in the NCCN guideline for NSCLC (n=2,106), 57.7%, 15.4% and 27.0% received chemotherapy, ICI-based therapy and TKIs respectively, as the 1st LOT (Figure 1A). Baseline demographic and clinical characteristics of the three sub-populations are summarized in Table S1. 35% of the patients continued to a 2nd LOT after disease progression. We separated these patients into two groups, with one group of patients started systemic treatment prior to 2015, the year of the 1st ICI approval by FDA for the indication of aNSCLCs, and the remaining patients started treatment during or after 2015. Not only there is a significant difference in the 1st LOT (Figures 1B,1C), but subsequent treatment also evolved. In the patient population who started treatment before 2015 and also had a 2nd LOT, when chemotherapy was the 1st LOT, 69.1%, 5.1% and 25.7% of the patients received another regimen of chemotherapy, an ICI-containing regimen, or a targeted therapy respectively, as the 2nd LOT (Figure 1B). In contrast, among patients who started treatment during or after 2015, received chemotherapy in the 1st line and also had a 2nd LOT, these percentages shifted significantly toward more ICIs, with 26.3%, 65.8%, 7.8% receiving chemotherapy, ICI, a targeted therapy respectively, in the 2nd line setting (Figure 1C). We also observed a change of treatment patterns in patients treated with TKIs as the 1st LOT. In this patient population, of those who were able to receive a 2nd LOT after disease progression, more patients (68.1% vs. 51.9% in Figure 1C vs. Figure 1B) were given another TKI during or after 2015 as new generations of TKIs with improved efficacy and tolerability became available.
We further examined more details on treatment regimens for ICI-based therapy and chemotherapy. In 1,214 patients treated with 1st line chemotherapy, 874 (72%) received platinum-based therapy. In 324 patients treated with 1st line ICI-based therapy, 155 (48%) received ICI agents without chemotherapy (145 ICI single agent, 10 nivolumab/ipilimumab); 169 (52%) patients were treated with ICI-chemotherapy combination, and majority (150 of 169) were pembrolizumab-containing regimens. In 167 patients received 2nd line ICI after progressing on 1st line chemotherapy, 143 (86%) were treated with a single ICI agent; 22 (13%) were treated with ICI-chemotherapy combination (pembrolizumab-based or atezolizumab-based); 2 (1%) patients received nivolumab-ipilimumab combination.
Impact of treatment sequencing on survival outcomes
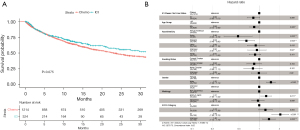
As expected, we observed significantly longer OS in patients who underwent 1st line ICI-based treatment, when compared with patients treated with 1st line chemotherapy as the reference group [HR = 0.83, log-rank P=0.075; adjusted HR (aHR) 0.82, P=0.066; Figure 2]. This result suggests OS in our data are consistent with what have been reported in randomized phase 3 trials. The multivariable Cox proportional hazard model also showed longer survival in the Asian and Hispanic population and less favorable outcomes for male patients (Figure 2B), consistent with what has been reported in the literature (23,24). Patients with an Eastern Cooperative Oncology Group (ECOG) score of 2 or greater had significantly shorter survival (aHR =2.06; P<0.001; Figure 2B).
To investigate the impact of treatment sequencing on clinical outcomes, we specifically assessed if sequencing of chemotherapy and ICI-based therapy impacted survival in those patients who were able to receive more than one LOT. We defined two patient populations: one received 1st line chemotherapy followed by ICI (n=167), and the other had 1st line ICI-based treatment followed by chemotherapy (n=37). No significant difference in OS was observed in both the Kaplan-Meier analysis (HR =1.49, log-rank P=0.20; Figure 3A) and multivariable Cox regression (aHR =1.36, P=0.39; Figure 3B). Next, we examined OS and TTNT, a real-world surrogate clinical endpoint for PFS, in patients who received ICI-based therapy in the 1st line (n=324) vs. 2nd or later line (n=229) setting; no significant difference was seen (Figure S2). We also compared OS in patients who had 1st line ICI or chemotherapy but were not able to receive any subsequent treatment. The 257 patients receiving 1st line ICI therapy had significantly longer OS than those patients given 1st line chemotherapy (HR =0.71, log-rank P=0.0049; adjusted aHR =0.71 in multivariable cox regression model, P=0.0070; Figure S3).

Effectiveness of 2nd line chemotherapy following 1st line ICI therapy
When the platinum doublet was the standard 1st line treatment for aNSCLC, patients were commonly offered docetaxel, pemetrexed or gemcitabine upon disease progression for subsequent treatment if they were physically fit. To evaluate the effectiveness of these 2nd line agents in the current ICI era following the new standard ICI-based 1st line treatment, we examined TTNT and OS in three patient populations treated with 1st line chemotherapy, 1st line ICI single agent or 1st line ICI-chemotherapy combination respectively, with all three groups received a 2nd line chemotherapy (Table S2). TTNT was similar among the 3 patient populations (Figure 4A). We incorporated a variable to account for different 2nd line regimens in the multivariable Cox proportional hazard model and reached the same conclusion (Figure 4B). OS (indexed to the starting date of the 2nd line therapy) was also similar in the three groups based on both Kaplan-Meier analysis and multivariable Cox regression (Figure 5A,5B). Notably, although statistical significance was not reached, the group with ICI-chemotherapy combination as the 1st LOT had numerically longer TTNT and survival than the chemotherapy 1st line group: median TTNT 11.4 vs. 8.6 months, aHR =0.73 (Figure 4A, blue vs. red curve); median OS not reached vs. 14.8 months, aHR =0.80 (Figure 5A, blue vs. red curve).


Discussion
Optimal treatment sequencing in cancer care is critical for achieving the greatest clinical benefit. This is best illustrated in a limited number of clinical trials on treatment sequencing in cancers with multiple options recommended for the 1st line treatment of metastatic disease. For example, abiraterone, enzalutamide and docetaxel are the preferred regimens for the 1st LOT of metastatic castration resistance prostate cancers (mCRPC). In a randomized phase 2 crossover study to determine the best sequence of using both abiraterone and enzalutamide (25), enzalutamide but not abiraterone demonstrated activity as the 2nd line novel androgen deprivation therapy; the sequence of abiraterone followed by enzalutamide led to a longer time to 2nd PSA progression than the opposite treatment sequence. However, for most cancers, it is not practical to conduct randomized clinical trial to evaluate different sequencing strategies. For NSCLC, the discussion of treatment sequencing in the literature has mainly centered around the sequential use of different TKIs targeting oncogenic alterations based on their ability to overcome resistance mutations (26,27). There are two recent publications presenting an overview of treatment landscape in NSCLC based on RWD (28,29), but how different sequencing patterns impacted survival was not investigated. To the best of our knowledge, this is the 1st comprehensive study of treatment sequencing in aNSCLCs, not only to delineate how treatment landscape has evolved, but more importantly to assess the impact of various sequencing patterns on clinical outcomes.
Previous studies by us (18) and by others (30) have shown patients receiving ICI in different settings of LOT had similar treatment duration as well as OS. Results from this study suggested that even though ICI-based therapy has become the new standard 1st line treatment, chemotherapy followed by ICI may achieve similar clinical benefit as the reverse sequence of ICI followed by chemotherapy (Figure 3), consistent with the notion that if a patient is able to receive an ICI-based regimen during the course of treatment, the therapy does not appear to have diminished activity when given at a later line than the front-line setting. This result is clinically meaningful. It is a typical scenario when patients are diagnosed with de novo metastatic NSCLC, reflex ordered testing of tumor molecular/genetic markers is performed to guide treatment strategies. The turnaround time (TAT) is approximately 14 days, a target recommended in College of American Pathologists (CAP)/International Association for the Study of Lung Cancer (IASLC)/Association for Molecular Pathology (AMP) molecular testing guidelines for NSCLC (31), but can be an issue for rural community practices where TAT may take up to more than 4 weeks (32,33). In a recent publication (34), it was reported that median TAT of DNA testing was 26 days in UK Welsh Thoracic Oncology Group. Furthermore, there are still challenges in low-and middle-income countries to access the contemporary treatment options including ICI (35). For many patients who are symptomatic and need immediate treatment for disease control, the treating oncologists may start platinum doublet before molecular testing results become available. The reason for not including an ICI in the initial treatment is that if the testing result identifies a sensitizing epidermal growth factor receptor (EGFR) mutation (thereby making osimertinib the appropriate 1st line therapy), using osimertinib after ICI can cause severe and life-threatening toxicity (36,37). If molecular testing reveals the tumor does not harbor an oncogenic driver mutation and the patient is responding well symptomatically to the initial cycles of platinum chemotherapy, the treating oncologist or the patient may elect to continue the therapy without adding an ICI for various reasons, such as concerns for immune-related adverse events or cost-effectiveness. Our study suggests that when the patient progressed on chemotherapy, an ICI agent can still be effective as the 2nd LOT. We should point out that our results are in contrast with randomized phase 3 studies where patients receiving 1st line ICI had longer OS than those receiving chemotherapy 1st line and later crossing over to ICI treatment (38-40). This could be due to the heterogeneous nature of RWD and more diverse patient population in the real-world clinical setting; therefore, our results should be interpreted in caution. Furthermore, since the majority of the patients with aNSCLC only receive one LOT (Figure 1), and ICI-containing regimens as the front-line therapy led to longer OS than chemotherapy (Figure 2, Figure S3), it is advisable to provide ICI-based therapy as the best treatment option in the front-line setting.
In oncology drug development, it is not uncommon that a new therapy is established as the 1st line treatment, but subsequent treatment options are based on clinical trials conducted when patients were treated with the previous standard 1st line therapy. Consequently, it is clinically important to confirm the effectiveness and to reevaluate efficacy-safety balance of the 2nd line regimens when the 1st line therapy has improved. RWD offer an ideal data source for such investigations. Other than NSCLCs, another example is hormone receptor-positive and HER2-negative breast cancers where cyclin-dependent kinase (CDK)4/6 inhibitors were incorporated into endocrine therapy several years ago, replacing endocrine therapy alone as the 1st LOT. A recent study of RWD has shown the everolimus-exemestane combination as a later line treatment option for metastatic hormone receptor-positive and HER2-negative breast cancers remains an effective treatment option, regardless of prior treatment with endocrine therapy alone or endocrine therapy combined with a CDK4/6 inhibitor (41). Our study represents the 1st investigation on the effectiveness of 2nd line chemotherapy for NSCLC in post-ICI settings, and the results suggest the 2nd line chemotherapy is still effective after 1st line ICI-based treatment (Figures 4,5). Interestingly, in patients who received an ICI-chemotherapy combination for 1st LOT, 2nd line chemotherapy resulted in numerically longer OS (indexed to the starting date of 2nd line chemotherapy) than the patients with chemotherapy as the 1st LOT (Figure 5A). This observation is similar to what has been reported in other cancers (42-44), implying ICI may enhance the efficacy of subsequent chemotherapy, even after disease progressed on ICI-based therapy.
We recognize there are significant limitations in our study. First, while RWD provides a rich source of clinical data pertaining to cancer diagnosis, treatment and clinical outcomes, the patient population is inherently heterogenous. Although we applied rigorous statistical methods to harmonize the data and to adjust for variables that may impact the results, there are other clinical characteristics, the “unknown unknowns”, that could impact response to treatment and survival, but were not accounted for in the analysis. The retrospective nature of the study is fundamentally different than prospective randomized trials in a well-controlled target population. Second, data completeness is another major issue of RWD. Variables such as smoking status (correlated with response to ICI) were incorporated into multivariable analyses. ECOG status is another critical variable associated with clinical outcomes. The percentage of patients with missing data in these variables is not insignificant. Of the 553 patients (Figure S2) received ICI in their treatment history, only 359 (65%) had PD-L1 data (237 positive and 122 negative). In our analysis of treatment sequencing (Figure 3), only 13.7% (167/1,214) and 11.4% (37/324) of patients received 2nd line treatment with ICI (after 1st line chemotherapy) and chemotherapy (after 1st line ICI), respectively. This is most likely due to the incompleteness of the data, because if patients were treated in a different clinic following the 1st line therapy at MSHS, the 2nd line treatment information are not available in our database. Third, clinical endpoints in RWD are limited. For most patients, there are no Response Evaluation Criteria in Solid Tumors (RECIST) or immune-related RECIST (irRECIST)-based assessment of radiographic response. PFS is typically not available for analysis. Even for OS, an objective survival measurement, it is not a trivial task to build an accurate and complete mortality database (45,46). Fourth, while reasons for treatment failure such as disease progression or adverse events would be valuable to analyze and share with scientific and clinical community, the information is not always captured in the RWD. Even for those patients with such information discussed in medical oncologists’ notes, manual curation of free-text notes can be resource consuming. Finally, this study is based on EMR of a single medical center. Although we began with 13,340 NSCLC patients in MSHS, there were only 2,106 patients had records of systemic treatment for advanced disease. The number becomes even smaller when we further identify patient subpopulations with specific treatment patterns. In our analysis of 2nd line chemotherapy (Figures 4,5), we are comparing two groups of patients (chemotherapy followed by chemotherapy, ICI-chemotherapy followed by chemotherapy) with only 158 and 25 patients in each group respectively. The small sample size underscores the necessity for replicating our findings in additional cohorts. Moreover, although the depth of treatment is an important aspect of cancer care, we do not have sufficient number of patients who received more than 2 lines of therapy to study the depth of treatment.
Conclusions
This study represents the 1st comprehensive analysis of treatment sequencing in aNSCLC. Even with the above-described limitations, results from this study with respect to the impact of sequencing on clinical outcomes and the effectiveness of post-ICI 2nd line chemotherapy are clinically meaningful and warrant further investigations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1481/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1481/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1481/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1481/coif). MKH and QP have been employees of the Sema4 Mount Sinai Genomics. The other authors were employees of the Sema4 Mount Sinai Genomics. DC also claims employment by Pfizer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was confirmed and approved by the Program for the Protection of Human Subjects at the Mount Sinai School of Medicine (IRB-17-01245).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813-24. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 2021;22:959-69. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Reck M, Gatzemeier U. Chemotherapy in stage-IV NSCLC. Lung Cancer 2004;45:S217-22. [Crossref] [PubMed]
- Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther 2016;16:653-60. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer 2019;125:4019-32. [Crossref] [PubMed]
- Ayers KL, Mullaney T, Zhou X, et al. Analysis of Real-World Data to Investigate the Impact of Race and Ethnicity on Response to Programmed Cell Death-1 and Programmed Cell Death-Ligand 1 Inhibitors in Advanced Non-Small Cell Lung Cancers. Oncologist 2021;26:e1226-39. [Crossref] [PubMed]
- Ayers KL, Ma M, Debussche G, et al. A composite biomarker of neutrophil-lymphocyte ratio and hemoglobin level correlates with clinical response to PD-1 and PD-L1 inhibitors in advanced non-small cell lung cancers. BMC Cancer 2021;21:441. [Crossref] [PubMed]
- Abernethy AP, Arunachalam A, Burke T, et al. Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting. PLoS One 2017;12:e0178420. [Crossref] [PubMed]
- McKay C, Burke T, Cao X, et al. Treatment Patterns for Advanced Non-Small-cell Lung Cancer After Platinum-containing Therapy in U.S. Community Oncology Clinical Practice. Clin Lung Cancer 2016;17:449-60.e7. [Crossref] [PubMed]
- Stewart DJ, Macdonald DB, Awan AA, et al. Optimal frequency of scans for patients on cancer therapies: A population kinetics assessment. Cancer Med 2019;8:6871-86. [Crossref] [PubMed]
- Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol 2009;4:1083-93. [Crossref] [PubMed]
- Pinto JA, Vallejos CS, Raez LE, et al. Gender and outcomes in non-small cell lung cancer: an old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018;3:e000344. [Crossref] [PubMed]
- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol 2019;20:1730-9. [Crossref] [PubMed]
- Itchins M, Lau B, Hudson AL, et al. ALK-Rearranged Non-Small Cell Lung Cancer in 2020: Real-World Triumphs in an Era of Multigeneration ALK-Inhibitor Sequencing Informed by Drug Resistance Profiling. Oncologist 2020;25:641-9. [Crossref] [PubMed]
- Kauffmann-Guerrero D, Kahnert K, Huber RM. Treatment Sequencing for Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer. Drugs 2021;81:87-100. [Crossref] [PubMed]
- Hess LM, Cui ZL, Li XI, et al. Treatment sequencing for the care of patients with advanced or metastatic non-small cell lung cancer in the United States. Curr Med Res Opin 2021;37:469-76. [Crossref] [PubMed]
- Stenehjem D, Lubinga S, Betts KA, et al. Treatment patterns in patients with metastatic non-small-cell lung cancer in the era of immunotherapy. Future Oncol 2021;17:2940-9. [Crossref] [PubMed]
- Khozin S, Carson KR, Zhi J, et al. Real-World Outcomes of Patients with Metastatic Non-Small Cell Lung Cancer Treated with Programmed Cell Death Protein 1 Inhibitors in the Year Following U.S. Regulatory Approval. Oncologist 2019;24:648-56. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018;20:129-59. [Crossref] [PubMed]
- Anand K, Phung TL, Bernicker EH, et al. Clinical Utility of Reflex Ordered Testing for Molecular Biomarkers in Lung Adenocarcinoma. Clin Lung Cancer 2020;21:437-42. [Crossref] [PubMed]
- Johnston KM, Sheffield BS, Yip S, et al. Comprehensive genomic profiling for non-small-cell lung cancer: health and budget impact. Curr Oncol 2020;27:e569-77. [Crossref] [PubMed]
- Cox S, Powell C, Morgan S. Implementing Genomic Testing for Lung Cancer Into Routine Clinical Practice - The Welsh Experience. Clin Oncol (R Coll Radiol) 2022;34:716-23. [Crossref] [PubMed]
- Pramesh CS, Badwe RA, Bhoo-Pathy N, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med 2022;28:649-57. [Crossref] [PubMed]
- Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 2019;30:839-44. [Crossref] [PubMed]
- Uchida T, Kaira K, Yamaguchi O, et al. Different incidence of interstitial lung disease according to different kinds of EGFR-tyrosine kinase inhibitors administered immediately before and/or after anti-PD-1 antibodies in lung cancer. Thorac Cancer 2019;10:975-9. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- Rozenblit M, Mun S, Soulos P, et al. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res 2021;23:14. [Crossref] [PubMed]
- Hadash-Bengad R, Hajaj E, Klein S, et al. Immunotherapy Potentiates the Effect of Chemotherapy in Metastatic Melanoma-A Retrospective Study. Front Oncol 2020;10:70. [Crossref] [PubMed]
- Harada D, Takata K, Mori S, et al. Previous Immune Checkpoint Inhibitor Treatment to Increase the Efficacy of Docetaxel and Ramucirumab Combination Chemotherapy. Anticancer Res 2019;39:4987-93. [Crossref] [PubMed]
- Liu YL, Zhou Q, Iasonos A, et al. Subsequent therapies and survival after immunotherapy in recurrent ovarian cancer. Gynecol Oncol 2019;155:51-7. [Crossref] [PubMed]
- Griffith SD, Miksad RA, Calkins G, et al. Characterizing the Feasibility and Performance of Real-World Tumor Progression End Points and Their Association With Overall Survival in a Large Advanced Non-Small-Cell Lung Cancer Data Set. JCO Clin Cancer Inform 2019;3:1-13. [Crossref] [PubMed]
- Griffith SD, Tucker M, Bowser B, et al. Generating Real-World Tumor Burden Endpoints from Electronic Health Record Data: Comparison of RECIST, Radiology-Anchored, and Clinician-Anchored Approaches for Abstracting Real-World Progression in Non-Small Cell Lung Cancer. Adv Ther 2019;36:2122-36. [Crossref] [PubMed]


