
Sequential therapy with crizotinib and a second-generation ALK inhibitor versus direct therapy of a second-generation ALK inhibitor in ALK-positive advanced lung cancer: a real-world study
Highlight box
Key findings
• There was no statistical difference in efficacy between crizotinib sequential second-generation ALK TKIs and direct therapy of second-generation ALK TKI regimens.
What is known and what is new?
• Several ALK TKIs have been identified as standard treatments for ALK rearrangement patients. Crizotinib sequential second-generation ALK TKIs or the direct therapy of second-generation ALK TKIs have both been confirmed to have good clinical efficacy
• In real-world studies, limited clinical studies have directly compared the two treatment options. We reviewed the real-world data of ALK+ lung cancer patients who received ALK TKI therapy.
What is the implication, and what should change now?
• The final PFS and OS analysis did not show statistical superiority of sequential therapy to direct therapy of second-generation ALK TIKs. The CNS effect of second-generation ALK TKIs was better than crizotinib.
• Prospective studies with larger samples are needed to validate the conclusions.
Introduction
Approximately 3−5% of patients with non-small cell lung cancer (NSCLC) have oncogenic rearrangements in the anaplastic lymphoma kinase (ALK) gene (1,2). Targeted drugs for ALK rearrangement can effectively treat this subtype of NSCLC.
Crizotinib was the first ALK tyrosine kinase inhibitor (TKI) approved by the US Food and Drug Administration as being more effective than standard chemotherapy for NSCLC (3-5). Previous studies showed that crizotinib produced an objective response rate (ORR) in approximately 65.0–75.5% of ALK rearrangement patients and was associated with a median progression-free survival (PFS) of 7.7–10.9 months (5-7). However, advanced ALK-positive patients using crizotinib are prone to central nervous system (CNS) metastasis, likely caused by poor penetration of the blood-brain barrier or acquired resistance (8,9). Several second-generation ALK TKIs have been developed to address these issues. Previous studies have demonstrated that sequential therapy with first-line crizotinib followed by alectinib has long-term benefits (10). Second-generation ALK TKIs provide a median PFS of 9.2–9.6 months in crizotinib-resistant patients (11,12). Studies have also shown that direct use of second-generation drugs can provide good survival benefits (6,13). The ASCEND-4 study showed PFS up to 16 months with first-line ceritinib (13). At present, both crizotinib and second-generation ALK TKIs (e.g., alectinib, brigatinib, and ceritinib) have been identified as the first-line standard treatments for ALK rearrangement patients (5,6,13). Many drugs are available for patients with ALK mutations. However, there is limited data on the clinical outcomes, long-term survival, and efficacy of different treatment sequences in Chinese patients. Also, how to better rank the administration sequence of ALK TKIs still requires further investigation.
The current retrospective study focused on exploring differences in efficacy between directly using second-generation ALK TKIs and using first-generation drugs followed by second-generation drugs. The goal was to provide guidance for clinical medication. In addition, a supplementary analysis was carried out to further explore the CNS efficacy with different sequences of ALK TKIs. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1783/rc).
Methods
Patients
A total of 556 lung cancer patients who harbored ALK rearrangement and who received targeted therapy at Zhejiang Cancer Hospital (Hangzhou, China) from May 2014 to October 2022 were screened (Figure 1). Of these patients, 115 patients who received crizotinib with sequential second-generation ALK TKIs and 96 patients who received a second-generation ALK TKI directly were enrolled. Patients were divided into sequential therapy and direct second-generation groups according to the treatment status of the targeted drugs.
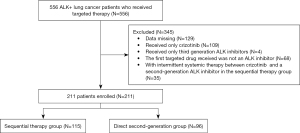
The major inclusion criteria were: (I) a documented ALK rearrangement by a positive result from fluorescence in situ hybridization (FISH) tests, ALK immunohistochemistry (IHC) (Ventana-D5F3), reverse transcriptase polymerase chain reaction (PCR), or next-generation sequencing (NGS); (II) use of ALK TKIs and complete survival data; (III) no intermittent systemic therapy between crizotinib and a second-generation ALK TKI in the sequential therapy group; (IV) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–2; (V) stage IIIB–IV; and (VI) at least one measurable lesion. The study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and was approved by the Ethics Committee of Zhejiang Cancer Hospital (IRB-2022-411). Written informed consent was waived due to the retrospective nature of the study.
Treatment and response evaluation criteria
Data on the patients with ALK rearrangement during the disease course were collected. Tumor responses were assessed by physical examination, routine laboratory tests, and imaging examination at 4- to 8-week intervals until progressive disease (PD) was identified. Two oncologists evaluated the tumor response based on the Response Evaluation Criteria in Solid Tumors 1.1.
The ORR was defined as the proportion of patients with complete remission (CR) plus partial remission (PR). The disease control rate (DCR) was defined as the proportion of patients with CR plus PR plus stable disease (SD). PFS was assessed from the first day of starting ALK TKIs to the earliest signs of disease progression or death owing to different causes. For evaluating duration for the sequential therapy group, we calculated the sum of the PFS with treatment that comprised of crizotinib and second-generation ALK TKIs, defined as ‘combined PFS’. The overall survival (OS) was calculated as the time between the date of confirmed advanced lung cancer and the death or last follow-up evaluation. CNS time to progression (TTP) with ALK TKIs was calculated from the start date of initial ALK TKIs in patients with intracranial lesions until CNS progression.
Statistical analysis and follow-up
SPSS (version 25.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 9.2.0; GraphPad, San Diego, CA, USA) were used to statistically analyze the data. The Chi-squared test or Fisher’s exact test was used to analyze the clinical classification variables. The survival analysis including PFS, OS, and CNS TTP in the various groups was calculated using the Kaplan-Meier method and compared by the log-rank test. Univariate and multivariate analyses were performed to identify independent risk and prognostic factors. Significance between groups was defined as P values <0.05, and two-tailed P values were calculated in all reports. The follow-up rate of the included patients was 100%. The total median follow-up time was 55.97 months (range, 49.85–62.09 months), including 66.07 months (range, 60.73–71.41 months) for the sequential therapy group and 28.23 months (range, 22.73–33.73 months) for the direct second-generation group. The last follow-up visit was on October 1, 2022.
Results
Patient characteristics
From May 2014 to October 2022, patients who received crizotinib with sequential second-generation ALK TKIs or directly received second-generation ALK TKIs at Zhejiang Cancer Hospital were enrolled. The baseline characteristics of the patients are summarized in Table 1. The median age of these patients was 54.0 (range, 28–81 years). Among them, 46.9% (99/211) were male, 88.2% (186/211) had an ECOG PS of 0–1, and 69.2% (146/211) had a smoking history. Sixteen (7.6%) patients were stage IIIB–IIIC stage and 195 (92.4%) patients were stage IV. Of these patients, ALK rearrangement was detected by FISH in seven (3.3%) patients, reverse transcriptase PCR in 24 (11.4%), Ventana IHC in 102 (48.3%), and NGS in 78 (37.0%) patients. Fifty-four (25.6%) patients were diagnosed with brain metastasis, 60 (28.4%) patients were diagnosed with bone metastasis, and 38 (18.0%) patients were diagnosed with liver metastasis. Previously, 48 (22.7%) patients had received surgery, 41 (19.4%) radiotherapy, and 70 (33.2%) chemotherapy. There was no statistical difference in baseline characteristics between the sequential therapy group and the direct second-generation group.
Table 1
| Characteristic | Patients, n (%) | Sequential therapy group, n (%) | Direct second-generation group, n (%) | P value |
|---|---|---|---|---|
| Age, years | 0.249 | |||
| Mean (standard deviation) | 53.4 (10.25) | 52.5 (9.9) | 54.6 (10.6) | |
| Median | 54.0 | 54.0 | 55.5 | |
| Range | 28–81 | 28–80 | 30–81 | |
| Sex | >0.999 | |||
| Male | 99 (46.9) | 54 (47.0) | 45 (46.9) | |
| Female | 112 (53.1) | 61 (53.0) | 51 (53.1) | |
| ECOG PS | 0.526 | |||
| 0 or 1 | 186 (88.2) | 103 (89.6) | 83 (86.5) | |
| 2 | 25 (11.8) | 12 (10.4) | 13 (13.5) | |
| Smoking history | 0.102 | |||
| Yes | 146 (69.2) | 74 (64.3) | 72 (75.0) | |
| No | 65 (30.8) | 41 (35.7) | 24 (25.0) | |
| Current stage of disease | 0.483 | |||
| IIIB-IIIC | 16 (7.6) | 7 (6.1) | 9 (9.4) | |
| IV | 195 (92.4) | 108 (93.9) | 87 (90.6) | |
| Histologic type | 0.416 | |||
| Adenocarcinoma | 204 (96.7) | 113 (98.3) | 91 (94.8) | |
| Squamous cell carcinoma | 4 (1.9) | 1 (0.9) | 3 (3.1) | |
| Other | 3 (1.4) | 1 (0.9) | 2 (2.1) | |
| ALK test method | 0.093 | |||
| FISH | 7 (3.3) | 4 (3.5) | 3 (3.1) | |
| Reverse transcriptase PCR | 24 (11.4) | 16 (13.9) | 8 (8.3) | |
| Ventana IHC | 102 (48.3) | 61 (53.0) | 41 (42.7) | |
| NGS | 78 (37.0) | 34 (29.6) | 44 (45.8) | |
| Brain metastasis | 0.752 | |||
| Yes | 54 (25.6) | 28 (24.3) | 26 (27.1) | |
| No | 157 (74.4) | 87 (75.7) | 70 (72.9) | |
| Bone metastasis | 0.445 | |||
| Yes | 60 (28.4) | 30 (26.1) | 30 (31.3) | |
| No | 151 (71.6) | 85 (73.9) | 66 (68.8) | |
| Liver metastasis | >0.999 | |||
| Yes | 38 (18.0) | 21 (18.3) | 17 (17.7) | |
| No | 173 (82.0) | 94 (81.7) | 79 (82.3) | |
| Previous surgery | 0.411 | |||
| Yes | 48 (22.7) | 29 (25.2) | 19 (19.8) | |
| No | 163 (77.3) | 86 (74.8) | 77 (80.2) | |
| Previous radiotherapy | 0.386 | |||
| Yes | 41 (19.4) | 25 (21.7) | 16 (16.7) | |
| No | 170 (80.6) | 90 (87.3) | 80 (83.3) | |
| Previous chemotherapy | 0.056 | |||
| Yes | 70 (33.2) | 45 (39.1) | 25 (26.0) | |
| No | 141 (66.8) | 70 (60.9) | 71 (74.0) |
ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group Performance status; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next-generation sequencing; PCR, polymerase chain reaction.
Efficacy and survival
The clinical characteristics of patients receiving ALK TKIs are summarized in Table 2. In the sequential therapy group, 0.9% (1/115) of patients who received crizotinib achieved CR, 67.0% (77/115) showed PR, 29.6% (34/115) showed SD, and 2.6% (3/115) had PD. The ORR was 67.8% and the DCR was 97.4%. In the direct second-generation group, 74.0% (71/96) of patients achieved PR, 24.0% (23/96) showed SD, and 2.1% (2/96) had PD. In these patients, the ORR was 74.0%, and the DCR was 97.9%. There was no significant difference in ORR (P=0.365) and DCR (P>0.999) between the treatment groups. For the entire efficacy of the sequential therapy group, the median combined PFS was 25.27 months [95% confidence interval (CI): 22.12–28.42], and the median OS was 70.27 months (95% CI: 51.08–89.46). For the efficacy of the direct second-generation group, the median PFS was 20.47 months (95% CI: 13.95–26.99), and the median OS was not reached. There was no significant difference between the treatment groups in PFS (P=0.644, Figure 2A) and OS (P=0.991, Figure 2B).
Table 2
| Characteristics | Sequential therapy group, n (%) | Direct second-generation group, n (%) |
|---|---|---|
| Line of initial ALK TKI | ||
| First | 70 (60.9) | 71 (74.0) |
| Second or more | 45 (39.1) | 25 (26.0) |
| Best response under initial ALK TKI | ||
| Complete response | 1 (0.9) | 0 (0.0) |
| Partial response | 77 (67.0) | 71 (74.0) |
| Stable disease | 34 (29.6) | 23 (24.0) |
| Progressive disease | 3 (2.6) | 2 (2.1) |
| Second-generation ALK TKI | ||
| Alectinib | 62 (53.9) | 76 (79.2) |
| Brigatinib | 16 (13.9) | 2 (2.1) |
| Ceritinib | 17 (14.8) | 0 (0.0) |
| Others | 20 (17.4) | 18 (18.8) |
| Reason for second-generation ALK TKI discontinuation | ||
| Progression | 85 (73.9) | 47 (49.0) |
| Toxicity | 0 (0.0) | 2 (2.1) |
| Death | 3 (2.6) | 3 (3.1) |
| Ongoing | 27 (23.5) | 44 (45.8) |
| Median PFS (months, 95% CI) | 25.27 (22.12–28.42) | 20.47 (13.95–26.99) |
| 1-year PFS rate | 85.22% | 71.2% |
| 5-year PFS rate | 21.6% | 37.6% |
| Median OS (months, 95% CI) | 70.27 (51.08–89.46) | Not reached |
| 1-year OS rate | 96.5% | 93.6% |
| 5-year OS rate | 55.55% | 58.2% |
ALK, anaplastic lymphoma kinase; CI, confidence interval; OS, overall survival; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
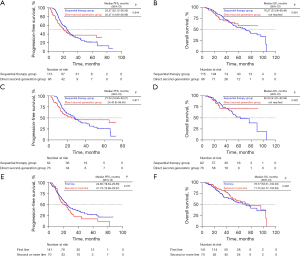
Separate analyses were performed in patients who used alectinib as the second-generation ALK TKI. In the sequential therapy group, 62 (53.9%) patients received alectinib after crizotinib progression. In the direct second-generation group, 76 (79.2%) patients were treated with alectinib directly. Among the 62 patients who received crizotinib in the sequential therapy group, 39 had PR, and 21 patients achieved SD, accounting for an ORR of 62.9% and a DCR of 96.8%. Among the 76 patients in the direct second-generation group, 57 had PR, and 18 achieved SD, accounting for an ORR of 75.0%. The DCR was 98.7%. There was no significant difference between the two groups in the ORR (P=0.140) and DCR (P=0.858) for patients using alectinib. For the sequential therapy group, the median combined PFS was 27.53 months (95% CI: 18.85–36.21), and the median OS was 62.50 months (95% CI: 41.61–83.39). For the direct second-generation group, the median PFS was 24.43 months (95% CI: 0–59.45), and the median OS was not reached. There was no significant difference between the treatment groups in PFS (P=0.871, Figure 2C) or OS (P=0.422, Figure 2D).
All patients were divided into two groups according to the initial ALK TKI: the first-line treatment group and the second- or more-line treatment group. Of these, 66.8% (141/211) of patients received an initial ALK TKI as first-line therapy. Among them, one had CR, 102 had PR, and 34 patients achieved SD, accounting for an ORR of 73.0%. The DCR was 97.2%. Overall, 33.2% (70/211) of patients received an initial ALK TKI as a second- or more-line treatment. Among them, 46 had PR and 23 achieved SD, accounting for an ORR of 65.7%. The DCR was 98.6%. There was no significant difference in the ORR (P=0.336) and DCR (P=0.879) for patients with different lines of initial ALK TKIs. For the first-line therapy treatment group, the median PFS was 24.60 months (95% CI: 19.64–29.56), and the median OS was 76.77 months (95% CI: 53.51–100.03). For the second- or more-line treatment group, the median PFS was 21.73 months (95% CI: 13.89–29.57) and the median OS was 71.70 months (95% CI: 42.77–100.63). There was no significant difference between the treatment groups in PFS (P=0.167, Figure 2E) and OS (P=0.446, Figure 2F).
CNS efficacy
A total of 54 patients had baseline brain metastases at study entry, of which 28 (24.3%) patients were in the sequential therapy group and 26 (27.1%) patients were in the direct second-generation group (Table S1). In the sequential therapy group, five patients (17.9%) who received crizotinib achieved CNS CR, two had CNS PD, and the overall CNS DCR was 92.9%. In the direct second-generation group, eight patients (30.8%) achieved CNS CR, none had CNS PD, and the overall CNS DCR was 100.0%. Among the 54 patients, 32 patients had measurable baseline brain target lesions; the CNS ORR was 53.3% (3 CR + 5 PR) in the sequential therapy group and 82.4% (3 CR + 11 PR) in the direct second-generation group (Figure 3A). There was no significant difference in CNS ORR (P=0.116) between the two groups. The CNS TTP for patients in the sequential therapy group and the direct second-generation group was 10.40 months (95% CI: 7.42–13.38) and 22.40 months (95% CI: 18.45–26.35), respectively. There was a significant difference in CNS TTP (P=0.040, Figure 3B) between the two groups.

Independent prognostic factors affecting PFS and OS
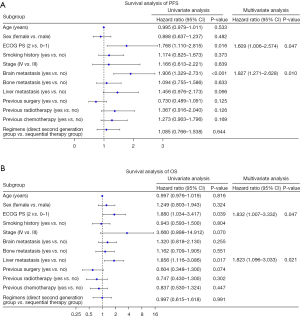
We performed a subgroup analysis of the direct second-generation group and the sequential therapy group based on baseline characteristics (Figure 4). The subgroup analysis showed no statistical difference in PFS or OS among the different populations. A Cox proportional hazard model was also performed to identify the prognostic factors for PFS and OS of the 211 ALK-positive patients. Univariate analysis indicated that poor PS and brain metastases were related to a poor PFS (Figure 5A). A poor ECOG PS score and liver metastases were related to a poor OS (Figure 5B). The statistically significant results of the univariate analysis were incorporated into the Cox multivariate regression model. Multivariate analysis showed that the ECOG PS score [hazard ratio (HR): 1.609; 95% CI: 1.006–2.574; P=0.047] and brain metastases (HR: 1.827; 95% CI: 1.271–2.628; P=0.010) were independent prognostic factors for PFS. Multivariate analysis showed that the ECOG PS score (HR: 1.832; 95% CI: 1.007–3.332; P=0.047) and liver metastases (HR: 1.823; 95% CI: 1.096–3.033; P=0.021) were independent prognostic factors for OS.

Discussion
Several ALK TKIs have been identified as standard treatments for ALK rearrangement patients in lung cancer. Crizotinib with sequential second-generation ALK TKIs or the direct therapy of second-generation ALK TKIs have both been confirmed to have good clinical efficacy (11-14). However, in real-world studies, limited clinical studies have directly compared the two treatment options. We reviewed the real-world data of lung cancer patients with ALK rearrangement who received ALK TKI therapy. Furthermore, the clinical benefits of ALK TKIs were retrospectively analyzed to evaluate the efficacy of different sequential ALK TKI therapies.
In previous clinical studies, both crizotinib with sequential second-generation ALK TKIs and the direct use of second-generation ALK TKIs produced good clinical efficacy. In the ALEX study, treatment-naïve advanced ALK-positive NSCLC patients who received alectinib achieved a PFS of 25.7 months (6). Zou et al. (10) found that first-line crizotinib with sequential alectinib demonstrated long-term benefits. The period from the start of crizotinib to the complete discontinuation of alectinib due to any cause was 39.2 months and the estimated 5-year OS was 68.6% in the overall population. In the J-ALEX study (15), a total of 103 patients were initially treated with alectinib, and 104 patients, including 78.8% of patients who received crizotinib followed by alectinib, were initially treated with crizotinib. Final OS analysis from J-ALEX did not show superiority of alectinib to crizotinib (68.6 vs. 68.0 months) (15). The WJOG9516L study (16) showed that the combined time-to-treatment failure of crizotinib followed by alectinib was significantly longer than in patients whose first-administered ALK TKI was alectinib (34.4 versus 27.2 months, P=0.004). However, the OS benefit of sequential therapy compared with therapy comprising initial treatment with alectinib was not shown (53.6 months vs. not reached, P=0.777). As per these data, it can be seen that second-generation ALK TKIs after crizotinib failure or the direct therapy of second-generation ALK TIKs both showed clinical benefit. The OS analysis data from previous clinical studies did not show superiority of direct therapy of alectinib compared with therapy of crizotinib followed by alectinib. However, the combined PFS with crizotinib and sequential second-generation ALK TKIs was superior to the initial treatment with second-generation ALK TKIs. However, real-world direct comparison data is limited, and the conclusion still needs further confirmation. We further performed a comparison of these two treatments. The OS results were similar to previous studies. Patients who received sequential therapy did not survive longer than patients in the direct therapy group (70.27 months vs. not reached, P=0.991). Compared to the WJOG9516L study, the final PFS analysis in our study did not show statistical superiority of sequential therapy to the direct therapy of second-generation ALK TIKs (25.27 vs. 20.47 months, P=0.644). This result most likely owed to the relatively small sample size of the study.
Some biomarkers may be associated with the prognosis of ALK-positive patients. Earlier studies have reported that the efficacy of ALK TKIs may be related to the type of echinoderm microtubule associated protein-like 4 (EML4)-ALK variants. NSCLC patients with EML4-ALK V1 respond to crizotinib with increased PFS after treatment (17,18). However, EML4-ALK V3-positive NSCLC patients demonstrate a higher metastatic spread and increased aggressiveness of the disease, while in vitro NSCLC cells harboring EML4-ALK V3 exhibit resistance to various ALK TKIs (19,20). Our previous research evaluated the effect of fusion heterogeneity on targeted therapy. The results showed that patients with tumors harboring multiple EML4-ALK isoforms had a statistically shorter median PFS and OS than patients with tumors harboring EML4-ALK single-isoforms when treated with crizotinib independent of the specific EML4-ALK variant type (21). Intratumoral EML4-ALK isoforms may predict the efficacy of targeted therapy in ALK-rearranged NSCLC (21). B-cell chronic lymphocytic leukemia/lymphoma (Bcl-2)-like 11 (BCL2L11) (Bim) deletion polymorphism was found to be associated with poor clinical response to crizotinib in patients with ALK fusion-positive NSCLC (22). In addition, the poor survival prognosis of ALK-positive patients was also associated with TP53 co-mutations (23). Unfortunately, this current study did not further explore the effects of these biomarkers on efficacy and survival, which marks the limitation and a direction for future research.
Almost all patients invariably experience progression on crizotinib, and approximately 40% of the patients with ALK-rearranged NSCLC develop CNS metastasis as an initial site of progression (24). After experiencing progression, most patients were enrolled in a subsequent second-generation ALK TKI study. In previous studies, second-generation ALK TKIs showed better CNS activity (25). ALEX clinical trials showed that the time to CNS progression was significantly longer with alectinib than with crizotinib (6). Based on previous clinical data, it can be seen that second-generation drugs can effectively avoid or delay CNS metastasis, bringing considerable benefits to patients (26). We analyzed 54 enrolled patients with baseline CNS metastasis to further evaluate the real-world efficacy of first- and second-generation ALK TKIs in the treatment of ALK-positive lung cancer with CNS metastasis. The results showed that the efficacy of the second-generation ALK TKIs was better than that of the first-generation ALK TKIs (CNS TTP, 22.40 vs. 10.40 months, P=0.040). Combined with previous studies, second-generation ALK TKIs had lower toxicity and higher intracranial efficacy than crizotinib (27-29). Therefore, second-generation ALK TKIs may be a better choice than first-generation ALK TKIs.
Furthermore, univariate and multifactorial analyses showed that the ECOG PS score and brain metastases were independent prognostic factors for PFS. The ECOG PS score and liver metastases were independent prognostic factors for OS.
Some limitations exist in our study. First, the primary data were obtained retrospectively, which may influence some outcomes. The patients in the sequential group were generally from earlier years in the study period, while patients in the direct second-generation groups were from later years. This might have affected the follow-up and could have biased the results. Second, our study was single-centered, which may have led to sampling bias and survival analysis bias. Third, the sample size of ALK-positive patients was small. Fourth, we did not exclude the influence of biomarkers such as TP53, Bim deletion polymorphism, and types of EML4-ALK variants on PFS and OS. Prospective studies with larger samples are needed to validate the conclusions.
Conclusions
In our study, the final PFS and OS analysis did not show statistical superiority of sequential therapy to direct therapy of second-generation ALK TIKs. The CNS effect of second-generation ALK TKIs in the initial treatment was better than that of first-generation ALK TKIs in the initial treatment. The prognostic factors for PFS included PS and brain metastases, and for OS it included PS and liver metastases.
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding: This study was funded by the Medical Scientific Research Foundation of Zhejiang Province (No. 2022KY653).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1783/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1783/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1783/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1783/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Institutional Ethics Committee at Zhejiang Cancer Hospital approved this study (IRB-2022-411), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1539-48. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Muller IB, De Langen AJ, Honeywell RJ, et al. Overcoming crizotinib resistance in ALK-rearranged NSCLC with the second-generation ALK-inhibitor ceritinib. Expert Rev Anticancer Ther 2016;16:147-57. [Crossref] [PubMed]
- Castellanos EH, Horn L. Re-Evaluating Progression in an Era of Progress: A Review of First- and Second-Line Treatment Options in Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer. Oncologist 2016;21:755-61. [Crossref] [PubMed]
- Zou Z, Hao X, Zhang C, et al. Clinical outcome, long-term survival and tolerability of sequential therapy of first-line crizotinib followed by alectinib in advanced ALK+NSCLC: A multicenter retrospective analysis in China. Thorac Cancer 2022;13:107-16. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Yang Y, Zhou J, Zhou J, et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med 2020;8:45-53. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Hotta K, Hida T, Nokihara H, et al. Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer. ESMO Open 2022;7:100527. [Crossref] [PubMed]
- Ito K, Yamanaka T, Hayashi H, et al. Sequential therapy of crizotinib followed by alectinib for non-small cell lung cancer harbouring anaplastic lymphoma kinase rearrangement (WJOG9516L): A multicenter retrospective cohort study. Eur J Cancer 2021;145:183-93. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3383-9. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [Crossref] [PubMed]
- O'Regan JK. Missing: Empirical theories of phenomenal consciousness. Cogn Neurosci 2021;12:82-3. [Crossref] [PubMed]
- Sampson J, Richards MW, Choi J, et al. Phase-separated foci of EML4-ALK facilitate signalling and depend upon an active kinase conformation. EMBO Rep 2021;22:e53693. [Crossref] [PubMed]
- Song Z, Lian S, Mak S, et al. Deep RNA Sequencing Revealed Fusion Junctional Heterogeneity May Predict Crizotinib Treatment Efficacy in ALK-Rearranged NSCLC. J Thorac Oncol 2022;17:264-76. [Crossref] [PubMed]
- Zhang L, Jiang T, Li X, et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer 2017;123:2927-35. [Crossref] [PubMed]
- Costa DB. TP53 mutations are predictive and prognostic when co-occurring with ALK rearrangements in lung cancer. Ann Oncol 2018;29:2028-30. [Crossref] [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [Crossref] [PubMed]
- Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8. [Crossref] [PubMed]
- Zou Z, Xing P, Hao X, et al. Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases-a multicenter retrospective study. BMC Med 2022;20:12. [Crossref] [PubMed]
- Tang H, Jin L, Zhang Z, et al. Comparison of Clinical Efficacy of Alectinib Versus Crizotinib in ALK-Positive Non-Small Cell Lung Cancer: A Meta-Analysis. Front Oncol 2021;11:646526. [Crossref] [PubMed]
- Wang Y, Shen S, Hu P, et al. Alectinib versus crizotinib in ALK-positive advanced non-small cell lung cancer and comparison of next-generation TKIs after crizotinib failure: Real-world evidence. Cancer Med 2022;11:4491-500. [Crossref] [PubMed]
- Zeng Q, Zhang X, He S, et al. Crizotinib versus Alectinib for the Treatment of ALK-Positive Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Chemotherapy 2022;67:67-80. [Crossref] [PubMed]


