
Efficacy and safety of Sanfeng Tongqiao Diwan in the treatment of upper airway cough syndrome: a randomized, double-blind, placebo-controlled clinical study
Highlight box
Key findings
• In this study, Sanfeng Tongqiao Diwan significantly improved the cough, postnasal drip, nasal congestion, runny nose, and overall symptoms of patients with upper airway cough syndrome (UACS) in the test group according to the Leicester Cough Questionnaire in Mandarin-Chinese (LCQ-MC) score, with a total effective rate of 86.6%.
What is known and what is new?
• To our knowledge, there has been considerable progress and a theoretical basis has been established for the treatment of UACS with traditional Chinese medicine (TCM). Sanfeng Tongqiao Diwan has shown the potential to alleviate acute, recurrent, and chronic rhinitis in adults.
• Our study demonstrated that Sanfeng Tongqiao Diwan alleviates the symptoms of UACS, improves the quality of life (QoL) of patients with UACS, and has good safety.
What is the implication, and what should change now?
• The results represent rigorous clinical evidence for the application of Sanfeng Tongqiao Diwan and may further support new TCM options in UACS treatment.
Introduction
Upper airway cough syndrome (UACS) refers to a clinical syndrome characterized by chronic cough as the main manifestation due to nasal disease-causing secretions flowing into the back of the nose and throat, directly or indirectly stimulating cough receptors. The main underlying diseases are allergic rhinitis and chronic rhinosinusitis, but UACS may also be related to throat disorders. The main clinical manifestations of UACS are cough, expectoration, and symptoms in the nose and pharynx (1). In Western medicine, etiological treatment and symptomatic treatment are the main therapeutic approaches for UACS and can include anti-inflammatory, antiallergy, and anti-cough medications; expectorants; nasal irrigation; and other treatments. The clinical symptoms often recur. The treatment of UACS with traditional Chinese medicine (TCM) is still in the exploratory stage. Related research indicates that UACS belongs to the “cough” category, mostly due to pathogenic wind invading the lungs (2). The lungs and nose are heterogeneous and of the same category, their pathogenesis is the same, their diseases are caused by mutual interactions, and their symptoms are interreferential; therefore, treatment should be based on the principles of dredging collaterals, dispelling wind, promoting the lungs, and relieving asthma. Sanfeng Tongqiao Diwan contains Scutellaria baicalensis (Huang qin), Schizonepeta tenuifolia (Jing jie), Asarum sieboldii (Xi xin), and Notopterygium incisum (Qiang huo). It has the actions of clearing heat and dispelling wind, dispelling cold, and opening orifices and can significantly improve the symptoms of acute sinusitis and allergic rhinitis, with no drug-related adverse reactions being observed in related studies (3,4). In this study, 60 patients with UACS were selected as the research participants to evaluate the clinical efficacy and safety of Sanfeng Tongqiao Diwan in the treatment of UACS and to provide new approaches for the treatment of UACS with prepared TCM. We present the following article in accordance with the CONSORT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-433/rc).
Methods
Research participants
The patients aged 18–65 years with UACS treated at the Department of Allergy and Clinical Immunology of the First Affiliated Hospital of Guangzhou Medical University were enrolled. This was a randomized, double-blind (blind to the patients and the investigators), placebo-controlled trial established by The First Affiliated Hospital of Guangzhou Medical University-Yangtze River Pharmaceutical Group Co., Ltd. (Taizhou, China). Sixty patients were divided equally into a placebo group and experimental group using the block randomization method. The random sequence was generated by independent statisticians through SAS 9.4 software (SAS Institute, Cary, NC, USA). In the experimental group, Sanfeng Tongqiao Diwan (38 mg/20 pills) was taken 3 times a day with warm boiled water after meals (tid); the treatment period was 14 days. The placebo group was given a simulant granule, mainly composed of starch and dextrin. Food colorants and flavoring agents were added to mimic the color, fragrance, taste, and texture of Sanfeng Tongqiao Diwan pills. Both Sanfeng Tongqiao Diwan and the simulant were provided by Yangtze River Pharmaceutical Group Co., Ltd. (Taizhou, China), and the dosage was the same for both groups. For the purpose of successful blinding, the test drug and the placebo were packed in sealed box with a drug number printed on the outside. During the treatment period, the 2 groups were not given other antihistamines or TCMs, and dextromethorphan oral disintegrating tablet (30 mg) was prescribed as needed (tid). The study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University (No. GYFYY-2020-170) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all the patients.
Study protocol
During the whole research process, the participants were required to complete a series of questionnaires including symptom relief score, Visual Analogue Scale score (VAS), and Leicester Cough Questionnaire in Mandarin-Chinese (LCQ-MC). At baseline, clinical investigators obtained inform consent and recorded demographic and general characteristics, including age, gender, height, weight, medical history, chest X-ray, spirometry, bronchial provocation test, allergen skin prick test, and induced sputum was performed as standard investigation in all patients. At baseline and treatment end point (15 days), laboratory and imaging examinations were conducted, including routine blood testing, liver and kidney function test, and 12-lead electrocardiogram (ECG) to evaluate safety. In addition, vital signs and adverse events (AEs) were monitored throughout the study. The primary outcome was total effective rate. The secondary outcomes included clinical efficacy, the change of VAS of clinical symptoms, and LCQ-MC scores before and after the treatment. If AEs occurred, the clinical investigators were required to record the symptoms and signs, abnormal laboratory indicators, the time of onset and end, the relationship with the test drug, the severity, the intervention, and prognosis of the event.
Eligible criteria
Patients who were diagnosed with UACS were eligible to participate in the trial if they were 18–65 years old and signed the informed consent form. The following diagnostic criteria for UACS were obtained from the Chinese National Guideline on Diagnosis and Management of Cough (2021) as revised by the Asthma Group of the Chinese Thoracic Society and the epidemiologic research on causes of chronic cough in China (5,6): (I) episodic or persistent cough for at least 8 weeks, mainly during the day, with fewer events after falling asleep, accompanied by nasal obstruction, runny nose, dry pharynx with foreign body sensation, and repeated clearing of the pharynx; (II) clinical manifestations and medical history of nose or (and) throat diseases; (III) auxiliary examinations supporting the diagnosis of nose or (and) throat diseases, including obvious hyperplasia of the posterior pharyngeal follicles and mucous or purulent secretion or occasional cobble-like changes; (IV) normal spirometry without airway hyper-responsiveness; (V) normal eosinophil percentage in induced sputum; and (VI) cough resolving after etiological treatment.
Patients were excluded for the following reasons: (I) patients with chronic obstructive pulmonary disease (COPD), cough-variant asthma, gastroesophageal reflux cough, eosinophilic bronchitis, bronchiectasis, or congenital respiratory diseases and other diseases causing chronic cough; (II) patients with severe heart, liver, kidney, mental diseases, or tumors; (III) patients who were pregnant, lactating, or planning for pregnancy; (IV) patients with known allergies to the study drug or drug composition; (V) patients unable to self-administer the drugs, potentially affecting the efficacy evaluation, or patients who had participated in clinical trials of other drugs; and (VI) patients who were smokers or alcoholics.
Patients could quit the study if they withdrew informed consent, or had adverse events and did not want to continue participating in the study, or did not comply with the protocol and/or the investigator’s instructions.
Primary efficacy indicators
Symptom relief score
The total curative effect of UACS symptoms was calculated using the nimodipine method as follows: total curative effect index = (pretreatment score − posttreatment score)/pretreatment score × 100%. “Completely recovered” was defined as the disappearance of clinical symptoms and signs, and the curative effect index was ≥95%; “significantly effective” was defined as significantly improved clinical symptoms and signs, and the curative effect index was ≥70%; “effective” was defined as improved clinical symptoms and signs, and the curative effect index was ≥30%; and “ineffective” was defined as no improvement or even aggravation of clinical symptoms and signs, and the curative effect index was <30%.
VAS score
A straight 10-cm line with a mark every 1 cm was used to assess the severity of the main symptoms, namely, cough, sputum, postnasal drip, nasal congestion, and runny nose: 0 indicated no symptoms, and 10 indicated the most severe level of symptoms. The higher the value was, the more severe the symptoms.
LCQ-MC score
This scale includes 3 dimensions: social function, physical condition, and psychological function. There are a total of 19 items, each of which is scored on a scale that ranges from 1 to 7 points. The scores of 3 dimensions are summed to give a total score. The higher the total score was, the better the quality of life (QoL) (7).
Routine blood and biochemical tests
For both groups, blood was collected before and after treatment. Within 1 hour of collection, 1 mL of whole blood was placed into a K2EDTA vacuum tube (BD Biosciences, Franklin Lakes, NJ, USA), and leukocytes, erythrocytes, eosinophils, and other cells were detected with a Beckman Coulter LH750 (Beckman Coulter, Brea, CA, USA). Then, 5 mL of blood was placed into a blood collection tube containing heparin anticoagulant, allowed to stand for 30 minutes, and centrifuged at 1,200 ×g for 10 minutes at 20 ℃. The supernatant was collected, and a reagent (AU5800 analyzer, Beckman Coulter) was used to assess renal function and liver function.
Routine urine tests
Before and after treatment, urine was obtained from the participants in the 2 groups. A total of 5 mL fresh midstream urine was collected, and within 1 hour, urine biochemical analyses were conducted using a Sysmex UF1000i (TOA Medical Electronics, Kobe, Japan).
Statistical methods
Based on a 2-sided significance level of 0.05, 27 participants in each group could achieve 90% power to detect a difference between the group proportions of 40% in total efficacy rate. The test statistic was the Z test with unpooled variance. The total sample size was 30 patients per group according to a 10% shedding rate.
All analyses were based on the intention-to-treat (ITT) principle. The full analysis set (FAS) included all patients who took medication and had postmedication evaluation at least once after randomization. Missing data were imputed by last-observation-carried-forward (LOCF) method in FAS. Patients without major protocol violations in FAS were included in the per protocol set (PPS). The safety set (SS) was composed of the patients who received study treatment and safety assessment at least once during the trial.
SPSS 20.0 statistical software (IBM Corp., Armonk, NY, USA) was used for data analysis. Categorical data including gender, number of underlying diseases, and clinical efficacy are expressed as the percentage of positive results and were analyzed with the chi-squared (χ2) test. The Newcombe-Wilson method was used to calculate the rate difference between 2 groups and its 95% CI. Measurement data including age, body mass index (BMI), disease duration, serum indicators of liver and renal function, cell counts of routine blood test, VAS, and LCQ-MC are expressed as the mean ± standard deviation (SD) or median (P25–P75) and were compared using independent-samples t-test or Mann-Whitney test based on the results of the Shapiro-Wilk test. Intragroup comparisons of measurement data before and after intervention were performed with a paired t-test or paired-samples Wilcoxon signed-rank test according to the results of normality test. A 2-sided P value <0.05 was considered statistically significant.
Results
Demographic data
A total of 60 patients with UACS from July 2021 to July 2022 were enrolled. In the first visit of the trial, 2 patients in the placebo group withdrew from the study because they failed to complete the blood test and refused to take any medication (Figure 1). There was no significant difference in age, sex, BMI, disease course, or underlying diseases between the 2 groups, and the data between the 2 groups were comparable (Table 1).
Table 1
| Variable | Experimental group | Placebo group | P value |
|---|---|---|---|
| Number of cases | 30 | 28 | |
| Age (years), mean ± SD | 33.7±4.2 | 32.5±3.9 | 0.549 |
| Sex, n (%) | 0.436 | ||
| Male | 17 (56.7) | 13 (46.4) | |
| Female | 13 (43.3) | 15 (53.6) | |
| BMI (kg/m2), mean ± SD | 21.2±2.3 | 20.8±1.7 | 0.403 |
| Disease duration (weeks), median [IQR] | 17 [13–21] | 15 [11–20] | 0.054 |
| History of underlying diseases, n (%) | 0.770 | ||
| Allergic rhinitis | 20 (66.6) | 18 (64.3) | |
| Chronic sinusitis | 6 (20.0) | 5 (17.9) | |
| Chronic pharyngitis | 2 (6.7) | 4 (14.3) | |
| Chronic tonsillitis | 2 (6.7) | 1 (3.6) |
UACS, upper airway cough syndrome; SD, standard deviation; BMI, body mass index; IQR, interquartile range.
Clinical efficacy
On the 15th day after treatment, the total effective rate for the experimental group was significantly higher than that for the placebo group, and the difference was statistically significant (79.6; 95% CI: 57.0 to 89.1; P<0.001; Table 2). Details about the symptom relief scoring are available in Table S1.
Table 2
| Clinical efficacy | Experimental group (n=30), n (%) | Placebo group (n=28), n (%) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Completely recovered | 1 (3.3) | 0 | 3.3 (−9.0, 16.7) | 0.330 |
| Significantly effective | 11 (36.7) | 0 | 36.7 (17.6, 54.5) | <0.001 |
| Effective | 14 (46.7) | 2 (7.1) | 39.6 (16.9, 57.5) | <0.001 |
| Total efficacy | 26 (86.7) | 2 (7.1) | 79.6 (57.0, 89.1) | <0.001 |
UACS, upper airway cough syndrome.
Comparison of the VAS scores for respiratory symptoms before and after treatment with Sanfeng Tongqiao Diwan
In the experimental group, the scores for nasal congestion, runny nose, cough, postnasal drip, and overall symptoms significantly decreased after treatment compared with those before treatment (all P values <0.05). The symptom scores in the placebo group did not change significantly after treatment. After treatment, the scores for nasal congestion, runny nose, cough, expectoration, postnasal drip, and overall symptoms were significantly lower in the experimental group than in the placebo group (all P values <0.05; Table 3).
Table 3
| Group | Time | Stuffy nose | Runny nose | Cough | Expectoration | Postnasal drip | General symptoms |
|---|---|---|---|---|---|---|---|
| Experimental group | Before treatment | 5.1±2.1 | 5.4±2.1 | 7.3±1.5 | 3.1±1.6 | 6.1±1.8 | 7.0±1.5 |
| After treatment | 3.7±1.5#* | 3.6±1.3#* | 3.8±1.2#* | 2.6±1.3 | 3.5±1.4#* | 3.8±2.0#* | |
| Placebo group | Before treatment | 4.9±1.6 | 5.2±1.8 | 6.7±1.0 | 3.1±1.9 | 6.0±1.1 | 7.3±1.2 |
| After treatment | 5.0±1.1 | 5.9±1.1 | 6.8±1.3 | 3.0±1.5 | 6.1±1.5 | 7.3±1.4 |
Data are presented as mean ± SD. Compared with the experimental group before treatment, #, P<0.001; compared with the placebo group after treatment, *, P<0.05. VAS, Visual Analogue Scale; UACS, upper airway cough syndrome.
Comparison of LCQ-MC scores before and after treatment with Sanfeng Tongqiao Diwan
Before treatment, there was no significant difference in LCQ-MC scores between the 2 groups. After treatment, the LCQ-MC score for the experimental group was significantly higher than that for the placebo group, and the difference was statistically significant (all P values <0.05; Table 4).
Table 4
| Group | Time | Physiological score | Social score | Psychological score | Total score |
|---|---|---|---|---|---|
| Experimental group | Before treatment | 30.7±5.5 | 19.8±2.5 | 26.3±5.2 | 78.8±7.9 |
| After treatment | 41.4±3.9#* | 24.0±2.9#* | 40.5±4.3#* | 109.6±6.5#* | |
| Placebo group | Before treatment | 31.4±4.1 | 21.6±3.4 | 26.8±5.5 | 80.9±8.1 |
| After treatment | 30.7±4.3 | 20.4±3.5 | 28.1±5.4 | 81.2±7.3 |
Data are presented as mean ± SD. Compared with the experimental group before treatment, #, P<0.001; compared with the placebo group after treatment, *, P<0.05. LCQ-MC, Leicester Cough Questionnaire in Mandarin-Chinese; UACS, upper airway cough syndrome.
Comparison of the liver and renal functions of patients before and after treatment
There was no significant difference in serum alanine aminotransferase (ALT), r-glutamyl transpeptidase (r-GT), blood urea nitrogen (BUN), or creatinine (Cr) between the 2 groups before and after treatment, and no AEs or serious adverse events (SAEs) occurred (Figure 2).
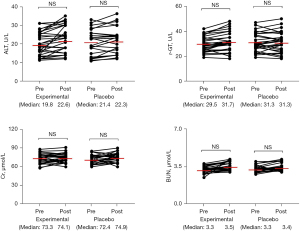
Comparison of routine blood test results for patients before and after treatment
There was no significant difference in leukocyte, hemoglobin, or platelet counts between the 2 groups before and after treatment, but the blood eosinophil count in the placebo group was significantly higher after treatment than before treatment (P=0.037; Figure 3).
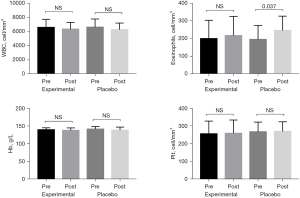
Discussion
This study found that single-drug treatment with Sanfeng Tongqiao Diwan significantly improved the clinical symptoms (cough, postnasal drip, and nasal congestion) and QoL of patients with UACS in the experimental group, but had no significant effect on liver function or renal function.
UACS is the most common cause of chronic cough, but its pathogenesis is still not completely clear. Currently, it is believed that the pathogenesis of UACS may involve a combination of multiple factors, including postnasal drip, airway inflammation, cough hypersensitivity, and neurogenic inflammation (8). Although there is no clear definition of UACS in TCM, UACS can be classified into the “cough”, “allergic rhinitis (Bi Qiu)”, and “nasal sinusitis (Bi Yuan)” categories on the basis of the symptoms, and the causes are summarized as wind, dampness, phlegm, heat, blood stasis, and deficiency. Among these, “pathogenic wind is the spearhead of all diseases” (9). Thus, the early onset of UACS is mostly caused by pathogenic wind invading the lungs. For the pathogenesis of UACS, pathogenic wind invades the lungs; the lungs lose their functions of diffusion, dispersion, purification, and descent; and fluid is not properly metabolized and stored, which over time condenses into phlegm and leads to cough. Therefore, clinical TCM treatment should follow the principles of dredging wind, opening orifices, clearing lungs, and clearing heat (10). Sanfeng Tongqiao Diwan clears away heat and removes dampness, dispels wind and cold, clears the orifices, and relieves pain, and the main ingredients are S. baicalensis, S. tenuifolia, A. sieboldii, and N. incisum. Modern medical research shows that S. baicalensis has antiallergic, antiviral, antioxidant, and other effects. S. baicalensis can downregulate the expression levels of various inflammatory mediators, effectively inhibit the release of mast cell mediators in mice, and relax airway smooth muscle (11,12). S. tenuifolia is suitable for treating symptoms such as fever, nasal congestion, runny nose, nasal mucosal congestion, and headache caused by exopathic wind-cold and heat stagnating in the lungs, and pharmacological studies have shown that S. tenuifolia acts on the nuclear factor-κB (NF-κB) and interleukin (IL)-17 pathways and has an inhibitory effect on histamine release (13,14). A. sieboldii has strong ascending and dispersing effects in the theory of TCM, possessing anti-inflammatory, anti-allergic, analgesic, and antipyretic properties (3,15). N. incisum is also a TCM with a pungent flavor and warm nature and has a propensity to the lung and kidney meridians. N. incisum expels wind, dispels cold, promotes fluid circulation, opens the orifices, has antiallergic and immunosuppressive effects, and is a commonly used formula for treating allergic rhinitis and asthma (16,17).
However, there have been no studies on the treatment of UACS with Sanfeng Tongqiao Diwan. In this study, Sanfeng Tongqiao Diwan significantly improved the cough, postnasal drip, nasal congestion, runny nose, and overall symptoms and significantly improved the QoL, as determined from the LCQ score of patients with UACS in the experimental group, with a total effective rate of 86.6%. In the experimental group, treatment was ineffective for 4 patients, potentially because of the presence of inflammation involving multiple paranasal sinuses in these patients, presenting a relatively wide scope of inflammation. For such patients, herbal medicine combined with conventional treatment may be more effective than herbal alone (18). The resolution of 2 cases in the placebo group may be related to taking dextromethorphan as needed. In addition, we reported no significant change of metabolic parameters and no clinically relevant variation regarding liver or kidney function. No drug-related side effect was observed during the treatment period in the 2 groups. A previous study reported that the major AEs could include mild rash, with an incidence rate of 3.3%, which resolved without any intervention (4). The increase in blood eosinophils in the placebo group after 2 weeks was likely related to uncontrolled inflammation. The eosinophils in the experimental group increased after treatment, but the difference was not statistically significant, suggesting that further research is warranted concerning the mechanism of action of Sanfeng Tongqiao Diwan against type 2 inflammation. The theoretical system of TCM is different from that of Western medicine, but specific groups of people who respond to treatment can still be identified through evidence-based medicine. For one, the clinical efficacy of TCM preparations is due to the synergistic effect of multiple TCMs. For another, a single drug has multiple action pathways (19). Therefore, the pharmacological effects of TCM preparations can be more complex than those of Western medicines.
The limitations of this study were the small sample size. Future related studies should include a larger sample size. In addition, the follow-up period was short, and the long-term treatment efficacy was not tracked and observed, and should thus be addressed in future studies.
Conclusions
Sanfeng Tongqiao Diwan significantly improved the cough, postnasal drip, and nasal symptoms of patients with UACS; improved QoL; and showed good safety. It thus represents a promising new option for the treatment of this condition.
Acknowledgments
Funding: This study was supported by the Open Project of State Key Laboratory of Respiratory Disease (No. SKLRD‐OP‐202004).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-433/rc
Trial Protocol: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-433/tp
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-433/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-433/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-433/coif). The authors have no conflicts of interest to declare.
Ethical Statement
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest 2006;129:63S-71S. [Crossref] [PubMed]
- Pan W, Zhao P. Observation of TCM Syndromes of 200 cases of upper airway cough syndrome. Chin J Information on TCM 2010;17:22-3.
- Hao Y, Huang W. Effect of sanfeng tongqiao dropping pill on acute sinusitis. Journal of Guizhou Medical University 2022;47:699-709.
- Hu H, Luo J, Ma J, et al. Efficacy and safety of Sanfeng Tongqiao Diwan in patients with allergic rhinitis: a single-arm clinical trial in China. Ann Transl Med 2022;10:684. [Crossref] [PubMed]
- Asthma Group of Chinese Thoracic Society. Chinese national guideline on diagnosis and management of cough (2021). Zhonghua Jie He He Hu Xi Za Zhi 2022;45:13-46. [PubMed]
- Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013;143:613-20. [Crossref] [PubMed]
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [Crossref] [PubMed]
- Lucanska M, Hajtman A, Calkovsky V, et al. Upper Airway Cough Syndrome in Pathogenesis of Chronic Cough. Physiol Res 2020;69:S35-42. [Crossref] [PubMed]
- Lin J, Chen W, Lin S, et al. Clinical effect of Jiawei xiao Qinglong soup in treating upper airway cough syndrome. Journal of Traditional Chinese Medicine 2021;20:57-9.
- Jiang H, Liu W, Li G, et al. Chinese Medicinal Herbs in the Treatment of Upper Airway Cough Syndrome: A Systematic Review of Randomized, Controlled Trials. Altern Ther Health Med 2016;22:38-51. [PubMed]
- Huang T, Liu Y, Zhang C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur J Drug Metab Pharmacokinet 2019;44:159-68. [Crossref] [PubMed]
- Zhao T, Tang H, Xie L, et al. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol 2019;71:1353-69. [Crossref] [PubMed]
- Eng YS, Lee CH, Lee WC, et al. Unraveling the Molecular Mechanism of Traditional Chinese Medicine: Formulas Against Acute Airway Viral Infections as Examples. Molecules 2019;24:3505. [Crossref] [PubMed]
- Liu C, Zhu Y, He Y, et al. Effect of Carbonized Herba schizonepetae on the Bioavailability of Eight Active Components from Pulsatillae Decoction in its Particular Adsorption and Release Performance. Curr Drug Metab 2021;22:957-68. [Crossref] [PubMed]
- Li Y, Gao C, Sha M. Research progress in pharmacological effects of Notopterygium incisum Ting. Journal of Liaoning college of Traditional Chinese Medicine 2002; 6:22-3.
- Lo PC, Lin SK, Lai JN. Long-term use of Chinese herbal medicine therapy reduced the risk of asthma hospitalization in school-age children: A nationwide population-based cohort study in Taiwan. J Tradit Complement Med 2020;10:141-9. [Crossref] [PubMed]
- Fei K, Hu X, Zhou L, et al. Progress in clinical application and pharmacological effects of Asarum sieboldi Mig. Acta Universitatis traditionis medicalis sinensis pharmacologiaeque Shanghai 2010; 24:87-90.
- Lee B, Kwon CY, Park MY. Herbal medicine for the treatment of chronic rhinosinusitis: A systematic review and meta-analysis. Front Pharmacol. 2022;13:908941. [Crossref] [PubMed]
- Ulbricht C, Chao W, Costa D, et al. Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab 2008;9:1063-120. [Crossref] [PubMed]
(English Language Editor: J. Jones)



