
C-reactive protein and lactate dehydrogenase serum levels potentially predict the response to checkpoint inhibitors in patients with advanced non-small cell lung cancer
Highlight box
Key findings
• CRP and LDH serum levels and their combination may predict the response to checkpoint inhibitors in patients with NSCLC.
What is known, and what is new?
• CRP and LDH serum levels are reported to correlate with different prognoses in NSCLC patients treated with checkpoint inhibitors.
• We established an association between CRP, LDH, and their combination with tumor response and prognosis in NSCLC patients treated with checkpoint inhibitors.
What is the implication, and what should change now?
• LDH, CRP, and their combination are potential predictive biomarkers of clinical response and need to be incorporated into future clinical trials.
Introduction
Lung cancer is a malignant tumor with high global morbidity and mortality. More than 50% of patients with lung cancer are diagnosed when the disease is already at an advanced stage (1). Chemotherapy is the standard treatment in most situations. Currently, the effective rates of platinum combination chemotherapy in patients with non-small cell lung cancer (NSCLC) range from 20% to 50% (2). Studies have pointed out that compared with chemotherapy and immunotherapy, immunocombination chemotherapy can further improve the quality of life and survival of patients with advanced NSCLC (3,4).
Although immunotherapy has some advantages, it does not show the same expected benefits for all patients. It was reported that only about 20–25% of NSCLC patients respond positively to immunotherapy (5). Therefore, further research is needed to identify patients who may benefit from immune checkpoint blockade therapy. Effective predictive biomarkers are necessary to achieve personalized treatment and guide clinical trial designs. In patients with advanced NSCLC, the evaluation of programmed cell death-ligand 1 (PD-L1) expression is used as an important biomarker for selecting patients suitable for anti-PD-L1 treatment (6). Generally, a high PD-L1 expression is predictive of immunotherapy benefits; however, some low-expression populations have also demonstrated benefits (7). Similarly, other potential biomarkers have failed to successfully predict the efficacy of immunotherapy (8). There are only a small number of cancers where mismatch repair, microsatellite instability, and tumor mutation can be used as biomarkers to predict the efficacy of immunotherapy (9).
Recently, research efforts in immunotherapy for lung cancer have begun to focus on the concentration of plasma inflammatory markers, such as lactate dehydrogenase (LDH) and C-reactive protein (CRP) (10-12). Although most inflammatory molecular markers are non-specific inflammatory markers, some studies suggest that they can participate in the occurrence and development of many kinds of malignant tumors. For example, one study suggested that increased preoperative CRP levels are associated with the inability to achieve complete resection in patients with NSCLC (13), and another study showed that changes in CRP in the early stage can predict response to checkpoint inhibitor treatment (14). It is suggested that systemic inflammatory response plays an important role in the occurrence and development of cancer and is related to the therapeutic effect for tumor patients. It has been reported that inflammatory markers have far-reaching effects on cancer development and the innate immune system (15), but the presumptive prognostic value of combined serum inflammatory molecular markers in NSCLC patients receiving immunotherapy has not yet been fully explored.
In this study, we analyzed the clinical significance of CRP and LDH to establish their association with tumor response and prognosis in patients with advanced NSCLC treated with checkpoint inhibitors. We present the following article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-240/rc).
Methods
Study design
The medical records of 116 patients with NSCLC were retrospectively reviewed from the 900th Hospital of the Joint Logistic Support Force from January 1, 2019, to March 1, 2022. The demographic data included gender, age, and smoking history. The clinical data included pathological type, gene mutation, granulocyte count, baseline CRP, baseline LDH, expression of tumor PD-L1, and objective response. Progression-free survival (PFS) was the primary endpoint and was assessed by investigators according to the Response Evaluation Criteria in Solid Tumors modified for immune-based therapeutics (iRECIST) (16). All baseline hematological parameters were obtained retrospectively 3 weeks before treatment as a result of normal medical practice.
Patients
Patients were included in the study if they had inoperable stage III–IV NSCLC confirmed by histopathology and were eligible to receive anti-PD-1/PD-L1 immunotherapy (single or combination regimen). All patients use at least one of the following immune checkpoint inhibitors (ICIs): Pembrolizumab, Tislelizumab, Sintilimab, Camrelizumab, Nivolumab, Toripalimab, Atezolizumab. The combination regimen is platinum-containing dual-drug chemotherapy, determined according to tumor histology. The treatment and outcome of each line were obtained. The Eastern Oncology Collaboration Group (ECOG) physical status score was 0–2 before taking treatment. Patients were excluded if they could not tolerate checkpoint inhibitors, had other primary malignant tumors, had received antibiotic therapy within the previous 3 weeks, or had been diagnosed previously with hematological or immune system diseases. The tumor stage was defined in accordance with the American Joint Committee on Cancer/International Union Against Cancer tumor-node-metastasis (TNM) classification system. Efficacy data were evaluated according to iRECIST. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of the 900th Hospital of the Joint Logistic Support Force, PLA (No. 2022-028). Individual consent for this retrospective analysis was waived.
Statistical analysis
X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA) was used to determine the optimal cutoff values of CRP and LDH. Patients were then divided into high and low groups. PFS was estimated using the Kaplan-Meier method, and the resultant curves were statistically tested by the log-rank method. Hazard ratios were estimated with the use of a stratified Cox regression model. All statistical test results were considered statistically significant at P<0.05. All statistical analyses were performed using SPSS Software version 25.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Characteristics of the 116 patients with stage III–IV NSCLC who received checkpoint inhibitors between January 2019 and June 2022 are shown in Table 1. They included 88 (75.9%) men and 28(24.1%) women. The median age of patients was 59.5 years (range, 26–78 years), and the median follow-up time was 5 months (range, 1–35 months). Of the 116 patients, 83 showed disease progression or died during follow-up. Overall, 35% of patients were current or former smokers.
Table 1
| Characteristic | Values |
|---|---|
| Age (years), median [range] | 59.5 [26–78] |
| Age (years), n (%) | |
| <60 | 66 (56.9) |
| ≥60 | 50 (43.1) |
| Gender, n (%) | |
| Male | 88 (75.9) |
| Female | 28 (24.1) |
| Smoking status, n (%) | |
| Never | 76 (65.5) |
| Current or former | 40 (34.5) |
| PD-L1 status, n (%) | |
| 0 | 11(9.5) |
| 0–50% | 8 (5.9) |
| >50% | 14 (12.1) |
| Histology, n (%) | |
| Squamous | 37 (31.9) |
| Non-squamous | 79 (68.1) |
| TNM stage, n (%) | |
| IIIB-IIIC | 9 (7.8) |
| IVA-IVB | 107 (92.2) |
PD-L1, programmed cell death-ligand 1; TNM, tumor-node-metastasis.
The optimum cut-point for CRP and LDH
The X-tile program was used to determine the cut-points for CRP and LDH and to assess statistical significance. According to the X-tile plots, the cut-points were as follows: CRP (8 mg/L) and LDH (312 U/L). These optimal cut-points most appropriately divided the cohort (Figure 1). We defined patients with CRP <8 mg/L as the low CRP group, CRP ≥8 mg/L as the high CRP group, LDH <312 U/L as the low CRP group, and LDH ≥312 U/L as the high LDH group.
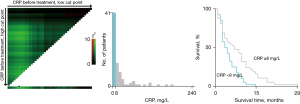
Efficacy of checkpoint inhibitors according to baseline CRP and LDH
We evaluated whether baseline CRP and LDH showed effects in PFS. A Kaplan-Meier analysis was used to evaluate every marker on patients’ survival. The results demonstrated high LDH and low CRP levels were associated with adverse PFS (Figure 2).
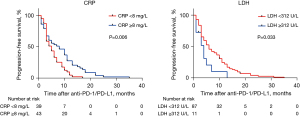
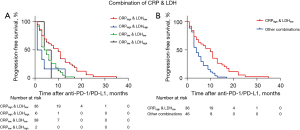
The predictive value of joint indicators: the combination of CRP and LDH
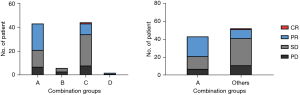
To further explore the value of CRP and LDH levels, patients were classified into four groups based on CRP (≥ and <8 mg/L) and LDH (≥ and <312 U/L) (Figure 2). A: CRPHigh with LDHLow (n=36); B: CRPHigh with LDHHigh (n=6); C: CRPLow with LDHLow (n=38); D: CRPLow with LDHHigh (n=2) (Table 2). The Kaplan-Meier survival analysis indicated statistical significance (P=0.002), as shown in Figure 3A. Patients with CRPHigh and LDHLow exhibited significantly better PFS than others. Therefore, we subsequently merged the four groups into two (CRPHigh with LDHLow versus others). The CRPHigh and LDHLow group had a significantly better objective response rate (ORR) and PFS than other combinations (ORR: 51.2% vs. 21.2%, and PFS: 10.0 vs. 5.3 months, P=0.007) (Figures 3,4).
Table 2
| Combinations | CRPHigh | CRPLow | Total |
|---|---|---|---|
| LDHHigh | 6 | 2 | 8 |
| LDHLow | 36 | 38 | 74 |
| Total | 42 | 40 | 82 |
CRP, C-reactive protein; LDH, lactic dehydrogenase.
Association between PFS and inflammatory molecular markers: univariate and multivariate Cox regression analysis
According to the results of univariate analysis, CRP (HR, 0.538; 95% CI: 0.334–0.866, P=0.011) and PD-L1 status (HR, 0.312; 95% CI: 0.130–0.747, P=0.009) showed a statistical significance (Table 3). Because some of the variables in the univariate analysis were covariates, we performed a multivariate analysis of gender, age, pathology, stage, smoking status, CRP, LDH, and PD-L1 status. In our cohort, only CRP was identified as a significant independent prognostic factor for PFS.
Table 3
| Characteristic | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Gender (male vs. female) | 1.028 | 0.644–1.643 | 0.907 | 1.130 | 0.173–7.389 | 0.899 | |
| Age (<60 vs. ≥60 years) | 0.874 | 0.580–1.315 | 0.518 | 1.437 | 0.424–4.870 | 0.560 | |
| Pathology (squamous vs. non-squamous) | 0.700 | 0.442–1.106 | 0.126 | 0.402 | 0.106–1.529 | 0.181 | |
| Stage (IIIB-C vs. IV) | 1.298 | 0.591–2.852 | 0.516 | 2.098 | 0.106–26.447 | 0.567 | |
| Smoking status (never vs. current or former) | 0.759 | 0.485–1.187 | 0.226 | 0.826 | 0.236–2.890 | 0.765 | |
| CRP (<8 vs. ≥8 mg/L) | 0.538 | 0.334–0.866 | 0.011 | 0.214 | 0.053–0.857 | 0.029 | |
| LDH (<312 vs. ≥312 U/L) | 1.942 | 0.995–3.789 | 0.052 | 2.822 | 0.820–9.716 | 0.100 | |
| PD-L1 status (0 vs. >0) | 0.312 | 0.130–0.747 | 0.009 | 0.771 | 0.213–2.788 | 0.692 | |
CRP, C-reactive protein; PFS, progression-free survival; LDH, lactate dehydrogenase, PD-L1, programmed cell death-Ligand 1; HR, hazard ratio; CI, confidence interval.
Discussion
For patients with advanced NSCLC, immunotherapy is approved as a first-line treatment. However, checkpoint inhibitors can cause serious adverse reactions (17,18), so it is particularly important to predict their efficacy. Although pathological subtypes, PD-L1 expression levels, tumor mutational burden (TMB) status, and microsatellite instability can predict checkpoint inhibitor efficacy (19), the detection methods of these biomarkers are complex and expensive (20). Therefore, it is particularly important to predict the effectiveness of checkpoint inhibitors using routine laboratory test indicators.
Inflammation is known to play an important role in tumor occurrence and development (21) and impacts the prognosis of NSCLC and tumor checkpoint inhibitors (22). Studies have shown that tumors can grow and evade immune surveillance by generating inflammatory and anti-inflammatory signals (23). Among them, inflammation-related markers, such as CRP and LDH, may play a key role in this process (24).
In this study, we found that baseline CRP and LDH levels had prognostic value in the immunotherapy of NSCLC. Further analysis using the optimal cut-off value (CRP: 8 mg/L; LDH: 312 U/L) demonstrated that patients with higher baseline CRP and lower baseline LDH showed better PFS than those with lower CRP and higher LDH levels. PD-L1 positivity alone was not an independent prognostic factor; possibly because blocking the PD-1 pathway did not affect the inflammatory microenvironment and lead to a T-cell response to cancer cells.
CRP is a classic and widely used serum indicator for evaluating the acute-phase response in the internal environment (25). Several studies have reported the significance of CRP as an important factor in predicting prognosis in NSCLC (26,27). LDH is the main enzyme in glycolysis and a classical indicator of tumor metabolism. It has previously been examined by researchers as a predictor of response to checkpoint inhibitors. Some studies have reported that an elevated serum CRP value was associated with inferior survival in NSCLC patients who received checkpoint inhibitors (28,29). However, the function of CRP in tumor progression and checkpoint inhibitor use remains controversial, and the unique role of various inflammatory factors remains unclear. One study reported that in the early stage of checkpoint inhibitor use, some patients with elevated CRP demonstrated a better prognosis (14), and other research has indicated that some inflammatory cytokines promote an anti-tumor response in the body (30). A recent study reported that the immune-inflamed tumor phenotype is strongly infiltrated with T-cells and is more often responsive to PD-L1 inhibitors (31) and that the cytokines released by T-cells may promote increased CRP levels. Other studies have found that CRP suppresses the response of T-helper cells (32,33) and delays tumor progression (34). Pyroptosis is an inflammatory form of cell death triggered by certain inflammasomes (35). Recent studies have shown that cytokines promoting the rapid synthesis of CRP, such as IL-1β and IL-18, are produced during pyroptosis and may enhance the efficacy of checkpoint inhibitors, including ICIs and CAR-T cells (36,37). CRP can also indirectly affect the efficacy of immunotherapy by affecting other prognostic markers, such as increase the PD-L1 expression in NSCLC, and improve the response of NSCLC to checkpoint inhibitors (38). Other reasons for the differences may be the difference in the population included in each study and the difference in the method and value of the optimal cut-off value of serum CRP. Some studies did not exclude patients with infection, and these patients usually combined with elevated CRP, and the prognosis is worse than normal patients.
To correct the inaccuracy of a single biomarker in predicting prognosis in patients with NSCLC, we combined LDH and CRP as joint indicators to provide a more accurate assessment of prognosis for these patients. To the best of our knowledge, this is the first study to investigate the use of combined CRP and LDH as a prognostic factor for checkpoint inhibitors. Our study demonstrated that patients in Group A (CRPHigh with LDHLow) had significantly better PFS and ORR than those in groups B, C, and D. This result suggests that the relationship between CRP and LDH in NSCLC immunotherapy needs further exploration. The limitations of this preliminary study were the limited sample size and the fact that the oncological results were retrospectively evaluated. Moreover, CRP is a non-specific parameter and is elevated in many situations, including infections, inflammatory diseases, tissue damage, and many other cancers (39). Furthermore, the molecular markers were all measured before treatment, and it is unclear whether subsequent changes have a role in prognostic assessment. Nevertheless, it cannot be ignored that these biomarkers have the advantage of being affordable and easy to access.
Conclusions
In this study, our data suggest that patients with high CRP levels and low LDH levels show better survival with anti-PD-1/PD-L1 therapy. LDH, CRP, and their combination are potential predictive biomarkers of clinical benefits and need to be incorporated into future clinical trials.
Acknowledgments
Funding: This work was supported by the Fujian University of Traditional Chinese Medicine: Scientific Research Program of University Management (No. XB2022142) and the External Cooperation of Science and Technology Program of Fujian Province (No. 202210034).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-240/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-240/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-240/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-240/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of the 900th Hospital of the Joint Logistic Support Force, PLA (No. 2022-028). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Miyawaki E, Kenmotsu H, Shintani Y, et al. Efficacy of platinum agents for stage III non-small-cell lung cancer following platinum-based chemoradiotherapy: a retrospective study. BMC Cancer 2022;22:342. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Pérol M, Felip E, Dafni U, et al. Effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol 2022;33:511-21. [Crossref] [PubMed]
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015;5:2892-911. [PubMed]
- Festino L, Botti G, Lorigan P, et al. Cancer Treatment with Anti-PD-1/PD-L1 Agents: Is PD-L1 Expression a Biomarker for Patient Selection? Drugs 2016;76:925-45. [Crossref] [PubMed]
- Yang W, Lee KW, Srivastava RM, et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat Med 2019;25:767-75. [Crossref] [PubMed]
- McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 2021;32:661-72. [Crossref] [PubMed]
- Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362:eaar3593. [Crossref] [PubMed]
- Lim JU, Yoon HK. Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine 2021;138:155363. [Crossref] [PubMed]
- Ozawa Y, Amano Y, Kanata K, et al. Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol 2019;36:33. [Crossref] [PubMed]
- Fukuda S, Saito K, Yasuda Y, et al. Impact of C-reactive protein flare-response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J Immunother Cancer 2021;9:e001564. [Crossref] [PubMed]
- Ni XF, Wu P, Wu C, et al. Elevated serum C-reactive protein, carcinoembryonic antigen and N2 disease are poor prognostic indicators in non-small cell lung cancer. Asia Pac J Clin Oncol 2015;11:e22-30. [Crossref] [PubMed]
- Klümper N, Saal J, Berner F, et al. C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer 2022;10:e004024. [Crossref] [PubMed]
- Rakotoarivelo V, Lacraz G, Mayhue M, et al. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation With Obesity or Diabetes. EBioMedicine 2018;30:237-47. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Quach HT, Johnson DB, LeBoeuf NR, et al. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol 2021;85:956-66. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14:847-56. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Liu W, Deng Y, Li Z, et al. Cancer Evo-Dev: A Theory of Inflammation-Induced Oncogenesis. Front Immunol 2021;12:768098. [Crossref] [PubMed]
- Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer 2019;7:267. [Crossref] [PubMed]
- Moik F, Zöchbauer-Müller S, Posch F, et al. Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer. Cancers (Basel) 2020;12:1619. [Crossref] [PubMed]
- Drpa G, Sutic M, Baranasic J, et al. Neutrophil-to-lymphocyte ratio can predict outcome in extensive-stage small cell lung cancer. Radiol Oncol 2020;54:437-46. [Crossref] [PubMed]
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 2011;48:155-70. [Crossref] [PubMed]
- Deng TB, Zhang J, Zhou YZ, et al. The prognostic value of C-reactive protein to albumin ratio in patients with lung cancer. Medicine (Baltimore) 2018;97:e13505. [Crossref] [PubMed]
- Leuzzi G, Galeone C, Gisabella M, et al. Baseline C-reactive protein level predicts survival of early-stage lung cancer: evidence from a systematic review and meta-analysis. Tumori 2016;102:441-9. [Crossref] [PubMed]
- Heppt MV, Heinzerling L, Kähler KC, et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer 2017;82:56-65. [Crossref] [PubMed]
- Oya Y, Yoshida T, Kuroda H, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget 2017;8:103117-28. [Crossref] [PubMed]
- Zhang C, Zhang Z, Sun N, et al. Identification of a costimulatory molecule-based signature for predicting prognosis risk and immunotherapy response in patients with lung adenocarcinoma. Oncoimmunology 2020;9:1824641. [Crossref] [PubMed]
- Gerard CL, Delyon J, Wicky A, et al. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat Rev 2021;101:102227. [Crossref] [PubMed]
- Zhang L, Liu SH, Wright TT, et al. C-reactive protein directly suppresses Th1 cell differentiation and alleviates experimental autoimmune encephalomyelitis. J Immunol 2015;194:5243-52. [Crossref] [PubMed]
- Shen ZY, Zheng Y, Pecsok MK, et al. C-Reactive Protein Suppresses the Th17 Response Indirectly by Attenuating the Antigen Presentation Ability of Monocyte Derived Dendritic Cells in Experimental Autoimmune Encephalomyelitis. Front Immunol 2021;12:589200. [Crossref] [PubMed]
- Salazar Y, Zheng X, Brunn D, et al. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J Clin Invest 2020;130:3560-75. [Crossref] [PubMed]
- Fang Y, Tian S, Pan Y, et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother 2020;121:109595. [Crossref] [PubMed]
- Hsu SK, Li CY, Lin IL, et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021;11:8813-35. [Crossref] [PubMed]
- Wang YY, Liu XL, Zhao R. Induction of Pyroptosis and Its Implications in Cancer Management. Front Oncol 2019;9:971. [Crossref] [PubMed]
- Akamine T, Takada K, Toyokawa G, et al. Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: A comprehensive analysis of systemic inflammatory markers. Surg Oncol 2018;27:88-94. [Crossref] [PubMed]
- Matsuo N, Azuma K, Hattori S, et al. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti-PD-1 inhibitor. Int J Cancer 2019;144:1170-9. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


