Non-intubated video-assisted thoracic surgery: where does evidence stand?
Introduction
The term non-intubated (aka awake or tubeless) video-assisted thoracic surgery (NIVATS) refers to thoracic operations that are performed without general anesthesia (GA) and mechanical ventilation in spontaneously breathing subjects.
This goal can be achieved by different anesthesia protocols entailing adoption of regional anesthesia methods, which have been applied to different surgical scenarios ranging from simple management of pleural effusion or pneumothorax (PNX) to more complex procedures including anatomical lung resections, thymectomy, and even tracheal resections or sleeve lobectomy (1-4).
Performing thoracic operations without GA is not a new and some historical series were already published in the 1950s (5-7). This happened before the advent of the Carlens’ tube and one-lung ventilation, which offered the possibility to operate on a deflated and immobile lung thus opening the modern era of thoracic surgery and leading locoregional anesthesia to rapidly fall into disuse.
In recent years, NIVATS has been the subject of a renewed interest. The main reason lying behind such a reappraisal consists in an enlarging ground of knowledge on adverse effects related to GA and one-lung ventilation. At the level of lung parenchyma, these adverse effects can be summarized into the concept of ventilator associated lung injury (8-15), which has been shown to occur irrespective of the patients’ lung function (12,13) though proving more dangerous in patients with pre-existing pulmonary disease. Other well-known side-effects of GA and one-lung ventilation include—but are not limited to—induction of cardiac arrhythmias (16), transient hypoxemia, injury to liver and kidney, cognitive deterioration, and impairment in perioperative immunosurveillance (17). Mechanical airway injury secondary to double-lumen tube insertion should be also taken into account, even though the estimated incidence of airway laceration is extremely low (18).
The rationale of NIVATS is that avoidance of one-lung ventilation may help achieve a reduction in perioperative morbidity, particularly in subjects with poor cardiorespiratory performance. Accordingly, it is not surprising that most of the earliest NIVATS experiences consisted of small case-series dealing with management of patients with chronic respiratory failure or other comorbidity.
A paradigm shift is—however—being observed more recently and in some centers, adoption of NIVATS is being progressively extended to patients without any substantial risk factor for GA and one-lung ventilation.
Indeed, in a recent survey from the European Society of Thoracic Surgeons, 70% of responders believed that ideal candidates for NIVATS are patients with multiple comorbidities although it is worth noting that 20% of them affirmed to be also favorable to the use of NIVATS regardless of patients’ comorbidity profile (19). This strategy appears to be justified when taking into account benefits other than protection from postoperative complications, which include reduced hospital stay, better quality of recovery and lower procedure-related costs. Furthermore, a lesser perturbation of immune and endocrine system with a possible positive impact on long-term oncological outcomes has been also hypothesized.
The aim of this review is to sort out the available evidence on NIVATS with particular emphasis on some outcome domains including technical feasibility, postoperative complications, hospital stay, costs and surgical efficacy compared to equivalent procedures performed with GA and single lung ventilation (GAVATS).
Literature search
A literature search was conducted on more relevant web databases (PubMed, Scopus, Google Scholar). Specific Boolean query to browse for publications was set as follows: [(awake) OR (non-intubated) OR (tubeless) OR (local anesthesia)] AND [(VATS) OR (videothoracoscopy)]. Additional keywords pertaining to the separate areas of interest were added as appropriate. Only abstracts specifically addressing NIVATS as the main subject were selected for subsequent evaluation. Papers belonging to the categories of letter, comment and editorials were not included in the analysis. Review articles were considered to extrapolate substantial information or to retrieve additional relevant papers not previously found at the default research. Papers who dated back more than 10 years were excluded unless comparative results of NIVATS vs. GAVATS were provided. In order to avoid redundancy of data and duplicate information, most relevant literature findings were either reported in the text or summarized in tables as deemed appropriate.
Meta-analysis
A meta-analysis pertaining to difference in operative morbidity rate and hospital stay between NIVATS and GAVATS was also performed choosing as the main inclusion criteria the presence of comparative analysis of the results. Results were taken both on fixed- and random-effect model. Inconsistency level was measured according to the Higgins’s I2 test. The MedCalc Statistical Software version 16.2.0 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2016) was used for this part of the study.
Technical feasibility
The most peculiar aspect of NIVATS is that operations are conducted following creation of a surgical PNX with preservation of spontaneous ventilation, diaphragmatic contraction and in many instances of the coughing reflex. This physiological aspect together with an imbalanced distribution of the tidal volume between the lungs and a certain rebreathing of ventilated gases may raise questions on technical feasibility as well as on procedural safety in the event of intraoperative complications or sudden cardiorespiratory instability requiring rapid switching to GA and intubation.
The vast majority of studies on NIVATS reported high rates of good feasibility, even though no study offered a systematic evaluation based on objective measurements of the surgical performance such as ergonomics parameters or surgeons’ mental workload.
As far as procedural safety is concerned, no study compared the occurrence of intraoperative adverse events between NIVATS and equipollent GAVATS procedures. Therefore, crude data in this topic can just be extrapolated from existing case series.
In an analysis of most relevant NIVATS studies summing up a total of 1,441 patients (20), the overall conversion rate was 2.4% with an obvious divergence between minor-intermediate (1%) and major procedures (10%) (Table 1).
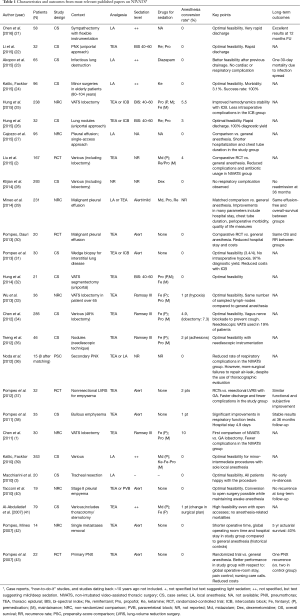
Full table
Most frequent causes of conversion were thick adhesions and respiratory movements, that accounted for some 41% of total events (overall incidence: 1.3%). Significant bleeding occurred in five cases only, as well as impairment in gas exchange (overall incidence for both events: 0.34%). Cardiac arrest occurred in one case only (<0.1%). Complications possibly attributable to anesthesiology maneuvers during contingency intubation have never been reported so far. These figures portray an acceptable safety profile of NIVATS in experienced hands, provided a careful selection of candidates.
It should be noted, however, that major NIVATS operations including lobectomy—which imply an increased risk of major intraoperative complications—are performed in high-volume thoracic surgery centers with an outstanding know-how with GAVATS. Given that no data on learning curves are available so far, it is difficult to establish an appropriate number of major GAVATS procedures to be recommended before embarking into a NIVATS program.
Operative morbidity
Reducing operative complications can be considered one of the most relevant goals of NIVATS. A systematic interpretation of available data in this regard is hard conducting due to a certain heterogeneity on methods for reporting and classifying the type and severity of complications. Published papers were found to be often underpowered when perioperative morbidity was not included amongst the primary outcome measures and treatment inclusion criteria were not standardized. Following, the most indicative literature findings are reported in separate subheads, according to the prevailing risk profile of patients.
Low-risk patients
In two separate studies dealing with peripheral lung nodules resections (44) and lung metastasectomy (42) published by Pompeo et al. (42,44) no difference in morbidity has been reported although a better oxygenation was observed on postoperative day one in both studies. In another small randomized trial by the same team (43), no substantial difference in morbidity was found in young, otherwise healthy subjects undergoing NIVATS or GAVATS blebectomy-pleuroabrasion for spontaneous PNX, despite few minor side-effects including vomiting and transient urinary retention occurred in the NIVATS group only.
A non-randomized comparison from Lesser and coworkers (45) analyzed the results of NIVATS resection of peripheral lung nodules with laser vs. stapled resection by GAVATS. A slight increase of prolonged air leak was found in the study group; however it is unclear as to whether these episodes were attributable to the different surgical technique rather than to the anesthesia method.
More recently the largest randomized-controlled trial (RCT) comparing NIVATS vs. GAVATS was reported by Liu and coworkers (2) who accrued a total of 354 patients with diverse thoracic diseases. Results were analyzed as a whole and in different treatment subgroups including pulmonary bullectomy, wedge resections and videothoracoscopic lobectomies. Overall postoperative morbidity rate was remarkably lower by NIVATS (6.7% vs. 16.7%, P=0.004). The main determinant of this finding was the reduction in respiratory complications (4.2% vs. 10.0%, P=0.039). In NIVATS patients, there were four adverse effects related to thoracic epidural (TEA) including back pain, dizziness, nausea and vomiting. In the control group, there were ten minor events attributable to orotracheal intubation. Differences in some surrogate indicators of overall quality of recovery including time to oral feeding, antibiotic administration and length of hospital stay were also found. Unfortunately, in this study, difference in perioperative morbidity was not the primary endpoint, which alongside with the lack of a power analysis in the design, suggest some caution in interpreting the results.
High-risk patients
A recent retrospective study from Klijian et al. (28) reported on up-to-date results of their experience with a total of 293 cases that were offered NIVATS under dexmedetomidine sedation. Despite comparison with a matched GAVATS group was not provided, the results are worth citing due to the large sample size. The cumulative postoperative morbidity rate was 4.3%. In particular, the most striking finding was that adverse events consisted almost exclusively of atrial fibrillation in patients receiving anatomical lung resection that accounted for an overall morbidity rate per subgroup of 32%. However, no respiratory complication occurred even though all patients who were offered lung resection had a very poor respiratory performance with a forced expiratory volume in one second of 1 Liter or less. This figure compares favorably with the overall incidence of major respiratory complications in the GAVATS lobectomy population (3.5%) (46).
In a small but elegant propensity-score matched analysis Noda and coworkers (36) compared the results of NIVATS vs. GAVATS for treatment of secondary PNX in patients with coexisting lung diseases. In this study the incidence of postoperative respiratory complications, including pneumonia and acute respiratory distress syndrome, was higher after GAVATS (P=0.02) although these patients had a relatively better performance status on admission.
Wu and coworkers (33) analyzed the results of a prospective, non-randomized comparative study of NIVATS vs. GAVATS lobectomy in geriatric stage I lung cancer patients (median age of 73 years). The majority of patients belonged to ASA III risk class due to the presence of relevant medical comorbidities other than advanced age. Surgical results and cumulative morbidity rates were similar in both groups. However, in GAVATS patients there was a remarkably higher incidence of postoperative delirium (4 vs. 0 events) and one life-threatening complication (pulmonary embolism) occurred in this group only.
A series of studies on adoption of NIVATS in lung-volume reduction surgery (LVRS) for severe emphysema have been published by our group. One of these studies entailed a RCT comparing the results of an original nonresectional LVRS technique performed in fully awake patients by NIVATS vs. resectional LVRS performed by GAVATS (37). A particular point of strength of this study was that both study groups received TEA. The overall morbidity rate was significantly lower in the NIVATS group (P=0.019) whereas one fatal event occurred in the GA group only. The incidence of prolonged air-leak, which is one of the most frequent adverse events after LVRS, was also lower in the NIVATS group as also shown in another non-randomized study (47).
Subjects with interstitial lung disease being scheduled for surgical biopsy are probably amongst those having the highest risk of severe ventilator-associated-lung-injury. Reported mortality rates in this subgroup range from 1.5% to 4.7% (48-50) and fatal events are mostly due to clinical exacerbation of the underlying disease.
A pilot study from our institution included 30 patients who were offered NIVATS biopsy of interstitial lung disease (31). Eight (28%) patients had a diffusion-capacity for carbon monoxide (DLCO) <40% predicted although a DLCO <30% predicted constituted an absolute exclusion criteria. Median operative time was 22 minutes and all patients tolerated well the surgical procedure with no relevant intraoperative impairment in gas exchange. Perioperative mortality was nil, and there was just one minor complication that accounted for an overall morbidity rate of less than 3%. These figures seems to justify the routine use of NIVATS in this setting particularly due to the easy feasibility of the surgical technique.
Further relevant contributions regard management of pleural diseases. Four studies compared NIVATS and GAVATS for the treatment of malignant pleural effusion and pleural empyema.
In a small single-center randomized study (33), complication rates was not reported although a significant difference was found in the amount of drainage fluid leading to shorter hospital stay in the NIVATS group.
In another 1 to 1 matched study (29) morbidity rate was analyzed in a total of 462 patients who underwent either talc pleurodesis through NIVATS by local anesthesia (LA) or GAVATS. The authors found a significant decrease in postoperative complications as well as in many other perioperative outcome measures including quality of life scores and hospital stay. Similar results came from Cajozzo et al. (27). Finally in a controlled study from Tacconi et al. (40), no substantial difference in morbidity rate was found with either NIVATS or GAVATS decortication for stage II pleural empyemas.
The results of two recent non-comparative studies are worthy reporting due to highly specific features of the treated patient population. Akopov et al. (23) employed NIVATS to manage serious pulmonary infections in a series of 65 patients, the vast majority of whom belonged to ASA 3 or 4 risk class. Overall morbidity rate was 13%, with all events consisting mostly of purely surgical complications including bleeding, chest wall phlegmon, and subcutaneous emphysema. One patient died from sepsis before postoperative day 30 due to failure to control the infection. On contrary, no major cardiovascular or respiratory complication was reported.
Katlic and Facktor (24) performed NIVATS for diverse pleural and pulmonary diseases in 96 patients with advanced age ranging from 80 to 104 years and reported a cumulative morbidity rate of 3.2%.
Figure 1 summarizes the results of the meta-analysis of most relevant comparative studies on this outcome domain. Data show, a mild and as yet not significant heterogeneity between studies (I2: 35.4%, P=0.1). The absolute risk difference of perioperative complications with NIVATS compared to GAVATS approached 10% under both fixed and random-effects assumption models (P=0.001).
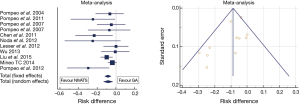
Hospital stay
Literature data appear to be rather concordant in indicating a global reduction in hospital stay duration after NIVATS as compared to GAVATS. Some caution is—however—required as in several studies standardized discharge criteria were not applied putting into question a potential observational bias.
Two out of the aforementioned randomized studies conducted by Pompeo et al.—dealing with talc pleurodesis (30) and LVRS (37) respectively—were powered according to the proportion of patients in whom an early discharge was achievable. In both these studies, a significantly higher proportion of patients who had received NIVATS operations could be discharged within postoperative day 3 or 6 respectively. The same research group also found a significant reduction in median hospital stay duration (2 vs. 3 days) in the setting of NIVATS resection of pulmonary nodules and primary spontaneous PNX (42,43). A quite similar observation came from the study of Lesser et al. (45).
Chen et al. (1) showed a non-significant trend toward shorter hospitalization after NIVATS lobectomy compared to GAVATS (5.9 vs. 7.1 days, P=0.07). In addition, in the previously mentioned study from Liu et al. (2) a significant reduction in hospital stay was reported in the subgroup of patients receiving NIVATS bullectomy and lobectomy, but not in that receiving wedge resection. Unfortunately, in this study standard deviations of results were not provided making impossible to include it in a pooled analysis (see below).
Other non-randomized studies reported significant differences in hospital stay in the settings of pleural effusion (27,40), pulmonary metastasectomy (42) and secondary PNX (36). On contrary, Wu et al. (33) in their study on NIVATS lobectomy in a geriatric population failed to demonstrate any difference in hospital stay length (33). Finally, in another study from our institution (51) NIVATS sympathectomy allowed all procedures to be performed in a 1-day surgery setting.
A meta-analysis of hospital stay results is shown in Figure 2. Mean difference in hospital stay between NIVATS and GAVATS was −0.53 days (random effect model; 95% CI: −0.74/−0.32, P<0.001). However, there was a relatively high inconsistency level (I2: 54%) reflecting a remarkable heterogeneity between included studies.
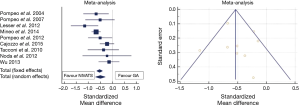
Long-term outcomes
So far, no follow-up data are available to investigate potential benefit or harm of NIVATS in oncological outcomes after resection for non-small cell lung cancer. Long-term outcome studies of thoracoscopic lobectomy showed that 5-year overall and cancer-specific survival rates were at least non-inferior to those achieved by an open approach (52). It is currently unclear as to whether these findings can apply to NIVATS procedures as well. However, there is no apparent reason to suspect a worse performance of NIVATS compared to GAVATS in guaranteeing similar results, given that equivalent surgical techniques for tumor resection and nodal dissection are deemed to be achievable (1,2,25,52).
No study addressed the question whether NIVATS may affect positively or negatively the escape of malignant cells from the primary tumor during surgical maneuvers. The possible role of better preserved anticancer immunosurveillance is also to be further evaluated, even though preliminary reports have suggested a lesser impairment in perioperative immune and hormonal status (53,54).
Data regarding other surgeries are somewhat sparse and fragmentary. Most relevant findings are summarized in Table 1, which suggests that NIVATS can be as effective as GAVATS in several surgical contexts, including surgical management of PNX, emphysema surgery, talc pleurodesis for malignant effusions and sympathectomy for palmar hyperhidrosis.
Procedure-related costs
Few studies focused on cost analysis have suggested that NIVATS can be associated with lower procedure-related costs than GAVATS. Overall, the main determinant of this result included shorter hospital stay, less need for drugs administration, avoided consumption of double-lumen tubes and other devices, and a global shortening of procedural times with an optimized planning of operating theatre workload. In particular, at our Institution significant reduction in hospital charges were achieved by employing NIVATS for surgical management of spontaneous PNX with an approximate saving per procedure of 1,000 Eurodollars (43), LVRS (800 Eurodollars) (37) and talcage for pleural effusion (750 Eurodollars) (30).
Finally, a further approximate cost saving of 200 Eurodollars per procedure was achieved also in patients undergoing NIVATS pulmonary biopsy for interstitial lung disease when intercostal block was used in place of TEA as the analgesia method (31).
Discussion
The present analysis shows a two-faceted scenario in terms of available clinical evidence supporting adoption of NIVATS.
On one hand, a pooled analysis of available data seems to confirm that NIVATS may help achieve a shorter hospital stay and a reduction in postoperative morbidity rate compared to GAVATS. This is particularly evident when dealing with patients at increased risk for GA, such as those with severe respiratory impairment and emphysema.
However, there is some uncertainty as to whether these findings can be generalized to all patients’ categories and all surgical contexts. In general, analyzed data suggest that in patients requiring minor procedures such as surgical management of spontaneous PNX, pleural effusion and empyema, results of NIVATS were at least equivalent to those achieved by GAVATS. Nonetheless when looking at adaptive physiology measures, several studies have shown a much faster normalization of arterial oxygenation after NIVATS (37,42-44) independent by the degree of respiratory impairment of the patients cohorts. Therefore, even if the indication to either strategy can be made on an individual basis, NIVATS might be preferred whenever GA appears to be a disproportionate tool when balanced against the simplicity of the surgery.
As well, our analysis points out the lack of sufficient evidence supporting the routine employ of NIVATS for major procedures including anatomical resection for lung cancer and other technically demanding operations.
Despite encouraging results, there are several potential biases to be taken into account. Besides a certain heterogeneity existing amongst studies on adopted primary outcome measures, most of published papers entail single-center studies coming from a restricted pool of highly-specialized University institutions investigating different aspects of NIVATS. Second, few studies have provided comparative analysis and even in those with intergroup comparisons, the samples sizes were generally small. Furthermore the pooled analysis of results might have been affected by the use of different analgesia methods and sedation protocols. For example, the use of TEA in NIVATS could have partially contributed to determine improved outcomes due to its analgesic and other-than-analgesic properties, even though the role of this anesthesia method in this setting is still controversial (55).
Hence, future investigations should be focused on stratified patients’ categories and should take into the account specific surgical scenarios with better defined methods, inclusion criteria and outcome measures including cost-effectiveness, self-perceived quality of perioperative recovery as well as long-term oncological outcomes when appropriate.
Another point of discussion is the great and rapidly evolving variability of adopted strategies to perform NIVATS, which might contribute to increase their attractiveness. In this respect as far as the anesthesia protocols are concerned, use of the bispectral index to titrate the level of sedation and maintain spontaneous ventilation, of intrathoracic vagal blockade to temporarily abolish the coughing reflex as well as the availability of novel drugs could all contribute to optimize the patients perioperative wellness and widen the spectrum of indications for NIVATS. From a surgical point of view, single-port access (22,26,32) as well as the availability of miniaturized and flexible instrumentation promise to further reduce the overall invasiveness of NIVATS (21,34,35).
In conclusion, NIVATS has been shown to reduce hospital stay and morbidity rate in selected cohorts. We expect that in a relatively short time span, available data will greatly increase both quantitatively and qualitatively eventually allowing to extrapolate more robust evidence about the real advantages and limitations of these ultra-minimally invasive surgical strategies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Macchiarini P, Rovira I, Ferrarello S. Awake upper airway surgery. Ann Thorac Surg 2010;89:387-90; discussion 390-1. [Crossref] [PubMed]
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg 2008;56:311-3. [Crossref] [PubMed]
- Vischnevski AA. Local anesthesia in thoracic surgery: lungs, heart and esophagus. Minerva Anestesiol 1954;20:432-5. [PubMed]
- Ossipov BK. Local anesthesia in thoracic surgery: 20 years experience with 3265 cases. Anesth Analg 1960;39:327-32. [Crossref] [PubMed]
- Buckingham WW, Beatty AJ. The technique of administering epidural anesthesia in thoracic surgery. Dis Chest 1950;17:561-8. [Crossref] [PubMed]
- Belperio JA, Keane MP, Lynch JP 3rd, et al. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 2006;27:350-64. [Crossref] [PubMed]
- Pavone LA, Albert S, Carney D, et al. Injurious mechanical ventilation in the normal lung causes a progressive pathologic change in dynamic alveolar mechanics. Crit Care 2007;11:R64. [Crossref] [PubMed]
- Licker M, Fauconnet P, Villiger Y, et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 2009;22:61-7. [Crossref] [PubMed]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [Crossref] [PubMed]
- Herndon B, Yagan M, Reisz G, et al. Metabolic and biochemical responses of the healthy human lung to nonthoracic surgery. Lung 2008;186:63-70. [Crossref] [PubMed]
- Pinheiro de Oliveira R, Hetzel MP, dos Anjos Silva M, et al. Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease. Crit Care 2010;14:R39. [Crossref] [PubMed]
- Zupancich E, Paparella D, Turani F, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg 2005;130:378-83. [Crossref] [PubMed]
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 2005;101:957-65. table of contents. [Crossref] [PubMed]
- Misthos P, Katsaragakis S, Theodorou D, et al. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardiothorac Surg 2006;29:591-5. [Crossref] [PubMed]
- Tønnesen E, Höhndorf K, Lerbjerg G, et al. Immunological and hormonal responses to lung surgery during one-lung ventilation. Eur J Anaesthesiol 1993;10:189-95. [PubMed]
- Miñambres E, Burón J, Ballesteros MA, et al. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg 2009;35:1056-62. [Crossref] [PubMed]
- Pompeo E, Sorge R, Akopov A, et al. Non-intubated thoracic surgery-A survey from the European Society of Thoracic Surgeons. Ann Transl Med 2015;3:37. [PubMed]
- Mineo TC, Tacconi F. From "awake" to "monitored anesthesia care" thoracic surgery: A 15 year evolution. Thorac Cancer 2014;5:1-13. [Crossref] [PubMed]
- Chen J, Lin J, Tu Y, et al. Nonintubated Transareolar Endoscopic Thoracic Sympathectomy with a Flexible Endoscope: Experience of 58 Cases. Ann Thorac Cardiovasc Surg 2016;22:12-9. [Crossref] [PubMed]
- Li S, Cui F, Liu J, et al. Nonintubated uniportal video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Chin J Cancer Res 2015;27:197-202. [PubMed]
- Akopov A, Egorov V, Deynega I, et al. Awake video-assisted thoracic surgery in acute infectious pulmonary destruction. Ann Transl Med 2015;3:100. [PubMed]
- Katlic MR, Facktor MA. Non-intubated video-assisted thoracic surgery in patients aged 80 years and older. Ann Transl Med 2015;3:101. [PubMed]
- Hung MH, Chan KC, Liu YJ, et al. Nonintubated thoracoscopic lobectomy for lung cancer using epidural anesthesia and intercostal blockade: a retrospective cohort study of 238 cases. Medicine (Baltimore) 2015;94:e727. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Chan KC, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014;98:1998-2003. [Crossref] [PubMed]
- Cajozzo M, Lo Iacono G, Raffaele F, et al. Thoracoscopy in pleural effusion--two techniques: awake single-access video-assisted thoracic surgery versus 2-ports video-assisted thoracic surgery under general anesthesia. Future Oncol 2015;11:39-41. [Crossref] [PubMed]
- Klijian AS, Gibbs M, Andonian NT. AVATS: Awake Video Assisted Thoracic Surgery--extended series report. J Cardiothorac Surg 2014;9:149. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Tacconi F, et al. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014;17:761-8. [Crossref] [PubMed]
- Pompeo E, Dauri M; Awake Thoracic Surgery Research Group. Is there any benefit in using awake anesthesia with thoracic epidural in thoracoscopic talc pleurodesis? J Thorac Cardiovasc Surg 2013;146:495-7.e1.
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Hsu HH, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Thorac Cardiovasc Surg 2014;148:e234-5. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [Crossref] [PubMed]
- Noda M, Okada Y, Maeda S, et al. Is there a benefit of awake thoracoscopic surgery in patients with secondary spontaneous pneumothorax? J Thorac Cardiovasc Surg 2012;143:613-6. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Pompeo E, Tacconi F, Frasca L, et al. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg 2011;39:1012-7. [Crossref] [PubMed]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Lesser TG. Laser application enables awake thoracoscopic resection of pulmonary nodules with minimal access. Surg Endosc 2012;26:1181-6. [Crossref] [PubMed]
- Sandri A, Papagiannopoulos K, Milton R, et al. Major morbidity after video-assisted thoracic surgery lung resections: a comparison between the European Society of Thoracic Surgeons definition and the Thoracic Morbidity and Mortality system. J Thorac Dis 2015;7:1174-80. [PubMed]
- Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg 2009;35:822-8; discussion 828. [Crossref] [PubMed]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg 2007;83:1140-4. [Crossref] [PubMed]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, et al. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg 2009;88:227-32. [Crossref] [PubMed]
- Ooi A, Iyenger S, Ferguson J, et al. VATS lung biopsy in suspected, diffuse interstitial lung disease provides diagnosis, and alters management strategies. Heart Lung Circ 2005;14:90-2. [Crossref] [PubMed]
- Elia S, Guggino G, Mineo D, et al. Awake one stage bilateral thoracoscopic sympathectomy for palmar hyperhidrosis: a safe outpatient procedure. Eur J Cardiothorac Surg 2005;28:312-7; discussion 317. [Crossref] [PubMed]
- Yamashita S, Goto T, Mori T, et al. Video-assisted thoracic surgery for lung cancer: republication of a systematic review and a proposal by the guidelines committee of the Japanese Association for Chest Surgery 2014. Gen Thorac Cardiovasc Surg 2014;62:701-5. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]
- Ke JD, Hou HJ, Wang M, et al. The comparison of anesthesia effect of lung surgery through video-assisted thoracic surgery: A meta-analysis. J Cancer Res Ther 2015;11 Suppl:C265-70. [Crossref] [PubMed]


