
Codeine prescription pattern and treatment responses in patients with chronic cough: a routinely collected institutional database analysis
Highlight box
Key findings
• Codeine prescription is frequent in real-world practice of patients with chronic cough, despite the lack of robust evidence or documentation of the efficacy and safety.
What is known and what is new?
• Codeine has been long used as an antitussive drug in several countries. However, a prescription pattern of codeine and efficacy has not been reported in detail.
• In the present retrospective cohort, 54.0% of patients referred to tertiary allergy and asthma clinics were prescribed codeine, and 14.0% of patients were prescribed codeine for >8 weeks. However, cough status change after codeine treatment was not documented in 38.7%. Side effects were described in 7.8%.
What is the implication, and what should change now?
• Our findings suggest a need for a stewardship program in guiding the appropriate use of codeine in the real world. Prospective studies are warranted to determine codeine treatment responses and safety.
Introduction
Cough is one of the most frequent symptoms for which patients seek medical care (1-3). Chronic cough, typically defined as a cough lasting >8 weeks in adults, is a prevalent condition that affects patient quality of life (4-6). Current guidelines for the management of chronic cough first recommend the identification of common cough-associated conditions, such as smoking, lung parenchymal diseases, asthma, eosinophilic bronchitis, rhinitis, or gastroesophageal reflux disease (5,7,8). Antitussive drugs are indicated in patients whose cough-triggering conditions are unclear, or in whom cough remains refractory to treatment (5,8,9).
Codeine has been long used as an antitussive drug in several countries. Earlier literature on the use of codeine for tuberculosis-associated cough dates back to approximately 200 years ago (10). In a recent retrospective administrative pharmacy and medical database analysis, antitussives including codeine were prescribed in 58.9% of chronic cough patients at specialist clinics in Southern California, US (11). The use of codeine- or hydrocodone-containing drugs was reported by 11.9% and 28.2% of chronic cough patients in community-based populations of South Korea and Taiwan, respectively (12). However, a prescription pattern of codeine, such as dose or treatment duration, has not been reported in detail. Furthermore, scientific evidence on the efficacy and safety is scanty, and such use was supported mainly by anecdotal experience but not by placebo-controlled clinical trials (13,14). Decades ago, several placebo-controlled trials evaluated the effects of codeine in patients with chronic cough (15-17), but the clinical impact was limited due to a very small sample size. The efficacy of opiate therapy was demonstrated in a placebo-controlled trial of low-dose, slow-release morphine in patients with chronic refractory cough (18); however, this was not a study of codeine. The European Respiratory Society guidelines recommended morphine for chronic refractory cough; however, morphine is not allowed for the use as an anti-tussive agent in most countries including South Korea. One randomized controlled trial found no benefits for codeine over placebo in patients with cough related to chronic obstructive pulmonary disease (19), but the trial did not include patients with refractory or unexplained chronic cough. Expert opinion suggests that opiates may be effective in <50% of patients (5); however, to our knowledge, codeine treatment responses and safety have not been formally reported, particularly in the real-world setting.
Routinely collected electronic health records (EHRs) contain comprehensive medical data, including information about health services, medical procedures, prescriptions, and diagnoses. The EHRs usually lack a disease-specific outcome measurement tool but have strength in evaluating prescription pattern and healthcare utilization. Based on a tertiary academic institutional EHR database in Seoul, Korea, we recently established a retrospective cohort of patients with chronic cough (3). Using the database, we evaluated codeine prescription patterns and clinical factors associated with codeine prescription and explored the treatment responses in chronic cough patients. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1857/rc).
Methods
Study participants and database
This was a retrospective analysis of patients with chronic cough who were newly referred to a tertiary allergy and asthma clinic (between July 2017 and July 2018). This was part of a previously reported cohort study of patients with chronic cough; the database and methodology (including patient selection and outcome measurements) has been described previously (3). Briefly, patients with chronic cough were identified using the search terms “cough” or “coughing” (either in English or Korean) in the EHR data field for the chief complaint, combined with the duration of the chief complaint (>8 weeks). Baseline parameters, including smoking history and cough-associated symptoms (throat abnormal sensation, productive sputum, dyspnea, wheeze, acid regurgitation, and postnasal drip), were retrieved from the structured EHR data. The data were recorded by specialist nurses and physicians (allergists or pulmonologists) at the clinics. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Asan Medical Center (IRB No. 2019-0511) and individual consent for this retrospective analysis was waived.
Codeine prescription patterns and responses
We retrieved drug prescription records during the first year after the initial outpatient visit. Codeine or codeine-containing antitussive drugs were the intervention of interest. The prescription records were analyzed for codeine prescription duration, mean daily dose, and 1-year cumulative dose. Patients were classified by codeine prescription duration: no codeine prescription; codeine prescribed for ≤8 weeks; codeine prescribed for >8 weeks.
Cough responses after codeine prescription was evaluated by manual EHR reviews. ‘Cough improved’ was defined by clear documentation of cough improvement or resolution within 1–4 weeks of starting codeine treatment (usually at the second outpatient visit after codeine prescription), because codeine treatment responses usually are observed rapidly (within 1–2 weeks) (13,18,20); we termed it as ‘improved’, but not as ‘responded’ because our analyses could not differentiate true codeine responses from placebo effects (21), spontaneous improvement, or effects of concomitant medication. ‘Not improved’ was defined by documentation of persistent cough or no improvement at the second outpatient visit after codeine prescription. Patients were classified as ‘unclear’ if they were lost to follow-up after codeine prescription or had no documentation of cough improvement in the EHR notes.
Healthcare utilization during the first year
Healthcare utilization, assessed according to data collected routinely during the first year after the initial outpatient visit, included medications, additional diagnostic workups, and the number of outpatient visits. We retrieved information on the use of additional diagnostic tests other than chest X-rays: pulmonary function tests, complete blood cell counts, sputum eosinophil counts, fractional exhaled nitric oxide (FeNO) tests, methacholine bronchial challenge tests, nasal endoscopy, laryngoscopy, and chest computed tomography (CT) scans.
Chest X-rays were considered abnormal if patients had bronchiectasis, tuberculosis, malignancy, or any other grossly abnormal parenchymal lesion identified by a radiologist. The pulmonary function parameters retrieved were forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). Type 2 (T2) inflammation was defined by positive sputum eosinophilia (≥3%), blood eosinophilia (≥300 cells/µL), or FeNO ≥30 parts per billion (ppb).
Drug records were analyzed for the following prescriptions: amitriptyline, antibiotics, leukotriene receptor antagonists, histamine H1-receptor antagonists, pseudoephedrine, proton pump inhibitors (PPIs), inhaled bronchodilators, inhaled corticosteroids (ICS), gabapentin/pregabalin, and oral corticosteroids (OCS). The total numbers of prescribed medications (other than codeine), additional diagnostic tests (other than chest X-rays), and outpatient visits were calculated.
Statistical analyses
The primary outcomes of the analyses were codeine prescription patterns and baseline clinical factors associated with codeine prescription. Descriptive data were presented as mean±standard deviation (SD), median with interquartile range (IQR), or percentages, depending on the type of distribution for each parameter. Codeine prescription patterns were displayed in histograms. The Chi-squared test for categoric variables, and the unpaired t-tests, Mann-Whitney test, or ANOVA for continuous variables, assessed between-group differences. Multinomial logistic regression analysis was performed to identify characteristics associated with codeine prescription, with adjustment for demographic factors (age and sex) and baseline parameters with P<0.05 in univariate analyses. All statistical analyses were performed using the Stata/SE 17.0 software package (Stata Corporation, College Station, TX, USA) or GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA, USA). All tests were two-sided, and P values were significant at <0.05.
Results
Codeine prescription patterns
Among a total of 1,233 patients newly referred with chronic cough, 666 (54.0%) were prescribed codeine at least once during the first year after the initial outpatient visit; 192 patients (15.6%) received codeine for <2 weeks, 301 patients (24.4%) for 2–8 weeks, and 173 patients (14.0%) for 8–52 weeks (Figure 1). Among patients prescribed codeine at least once, the mean (± SD) number of days on which codeine was prescribed was 51.4±66.8 (median 27.5; IQR 14–60 days); and the upper fifth percentile was 200 days (Figure 2A). The mean daily codeine dose prescribed was 32.2±16.5 mg/day (median 30; IQR 21.6–30.0 mg/day), and the upper fifth percentile was 60 mg/day (Figure 2B). The mean 1-year cumulative dose was 1,857.7±3,642.7 mg/year (median 720; IQR 420–1,800 mg/year), and the upper fifth percentile was 7,240 mg/year (Figure 2C).

Patient factors associated with codeine prescription
The baseline characteristics of patients were compared according to codeine prescription duration. Patients prescribed codeine for >8 weeks were older and had a longer cough duration than those prescribed codeine for ≤8 weeks or who did not receive codeine. The proportions of females and non-smokers were higher in both codeine groups (prescribed for ≤8 weeks and >8 weeks) than in the non-codeine group (Table 1).
Table 1
| Parameters | No codeine prescription (n=567) | Codeine prescribed for ≤8 weeks (n=493) | Codeine prescribed for >8 weeks (n=173) | P value |
|---|---|---|---|---|
| Codeine prescription | ||||
| Prescription duration (days) | 0 | 17 (14 to 30) | 100 (74 to 166) | <0.001 |
| Daily mean dose (mg) | 0 | 30 (20 to 30) | 30 (23.4 to 40) | 0.007 |
| 1-year cumulative dose (mg) | 0 | 510 (320 to 880) | 3,150 (2,220 to 5,600) | <0.001 |
| Demographic factors | ||||
| Age (years) | 54.5±16.3 | 53.8±15.3 | 58.9±14.1 | <0.001 |
| Cough duration (months) | 7 (3 to 24) | 5 (2 to 24) | 12 (4 to 60) | <0.001 |
| Female sex, % | 59.4 | 68.6 | 68.8 | 0.004 |
| Smoking, % | ||||
| Never | 68.0 | 75.7 | 77.3 | 0.001 |
| Former | 24.8 | 18.5 | 16.3 | 0.022 |
| Current | 7.2 | 5.8 | 6.4 | 0.726 |
| Associated symptoms, % | ||||
| Throat abnormal sensation | 65.6 | 74.4 | 85.0 | <0.001 |
| Productive sputum | 35.5 | 35.3 | 35.8 | 0.991 |
| Dyspnea | 21.5 | 15.0 | 17.3 | 0.022 |
| Wheeze | 20.6 | 20.7 | 22.5 | 0.855 |
| Acid regurgitation | 15.7 | 15.2 | 20.2 | 0.300 |
| Postnasal drip | 39.3 | 39.2 | 46.8 | 0.173 |
| Diagnostic test results | ||||
| CXR abnormality | 16.1 (72/448) | 15.2 (64/420) | 14.1 (21/149) | 0.835 |
| FEV1 (% of predicted) | 87.4±17.3 | 88.5±16.4 | 90.6±15.9 | 0.290 |
| FVC (% of predicted) | 86.1±14.1 | 86.2±13.2 | 86.3±12.0 | 0.992 |
| FEV1/FVC (%) | 78.9±10.3 | 80.7±9.3 | 80.3±8.8 | 0.090 |
| FeNO ≥30 ppb | 34.4 (11/32) | 20.2 (19/94) | 17.5 (7/40) | 0.067 |
| Blood eosinophilia (≥300 cells/µL) | 25.0 (38/152) | 26.3 (36/137) | 19.2 (9/47) | 0.603 |
| Sputum eosinophilia (≥3%) | 39.3 (35/89) | 35.2 (38/108) | 33.3 (10/30) | 0.773 |
| Any T2 positive | 29.4 (63/214) | 25.5 (64/251) | 24.4 (22/90) | 0.543 |
Data were presented as mean ± standard deviation, median (interquartile range), or % (n/n). CXR, chest X-ray; FEV1, forced expiratory volume in 1 s; FVC, forced volume capacity; FeNO, fractional exhaled nitric oxide; T2, type 2. T2 means that any one of FeNO ≥30 ppb, blood eosinophilia ≥300 cells/μL, and sputum eosinophilia ≥3% is satisfied.
Among associated symptoms, patients prescribed codeine for >8 weeks had a significantly higher rate of throat abnormal sensation than the other two groups [85.0% vs. 74.4% (codeine for ≤8 weeks) vs. 65.6% (no codeine); P<0.001] (Table 1). However, dyspnea was significantly more frequent in patients not prescribed codeine than in the other two groups (P=0.022). The results of baseline diagnostic tests, including chest X-ray, lung function, and T2 inflammatory markers (such as FeNO, and blood and sputum eosinophils), were not significantly different among the groups.
Multinomial logistic regression analysis was performed to examine baseline factors associated with codeine prescription (vs. no codeine), with adjustment for those factors with P<0.05 in Table 1. The probability of longer codeine prescription (>8 weeks) was significantly associated with older age, longer cough duration, non-smoker, and throat abnormal sensation (Table 2).
Table 2
| Parameters | No codeine prescription (n=567) (Ref.) | Codeine prescribed for ≤8 weeks (n=493) | Codeine prescribed for >8 weeks (n=173) | |||
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI)* | P value | Adjusted OR (95% CI)* | P value | |||
| Age (years) | 1.00 | 1.00 (0.99–1.01) | 0.737 | 1.03 (1.01–1.04) | <0.001 | |
| Female (vs. male) | 1.00 | 1.27 (0.92–1.75) | 0.154 | 0.92 (0.56–1.49) | 0.727 | |
| Cough duration (months) | 1.00 | 1.00 (1.00–1.00) | 0.957 | 1.00 (1.00–1.00) | 0.045 | |
| Smoking history (no vs. yes) | 1.00 | 1.26 (0.90–1.76) | 0.182 | 1.75 (1.04–2.94) | 0.036 | |
| Throat abnormal sensation (yes vs. no) | 1.00 | 1.52 (1.15–2.00) | 0.003 | 3.20 (2.01–5.09) | <0.001 | |
| Dyspnea (yes vs. no) | 1.00 | 0.67 (0.48–0.94) | 0.018 | 0.65 (0.41–1.04) | 0.075 | |
*, adjusted for age, sex, cough duration, smoking history, throat abnormal sensation, and dyspnea. Ref., reference; OR, odds ratio; 95% CI, 95% confidence interval.
Comparison of healthcare utilization according to codeine prescription duration
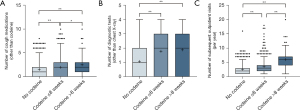
Codeine prescription duration was positively associated with the use of other cough-related medications, such as amitriptyline, antibiotics, gabapentin/pregabalin, H1RA, PPI, or pseudoephedrine (Table 3). Patients who were prescribed codeine also underwent significantly more diagnostic tests, such as chest CT scan, induced sputum, FeNO, nasopharyngoscopy, laryngoscopy, or methacholine bronchial challenge tests. The number of cough medications, diagnostic tests, or subsequent outpatient visits was significantly associated with codeine prescription and duration (Figure 3).
Table 3
| Parameters | No codeine prescription (n=567) | Codeine prescribed for ≤8 weeks (n=493) | Codeine prescribed for >8 weeks (n=173) | P value |
|---|---|---|---|---|
| Other cough-related medications, % | ||||
| Amitriptyline | 0.7 | 1.4 | 8.7 | <0.001 |
| Antibiotics | 11.5 | 18.1 | 20.2 | 0.002 |
| Gabapentin/pregabalin | 0.4 | 1.8 | 8.1 | <0.001 |
| H1RA | 30.7 | 47.1 | 59.5 | <0.001 |
| LTRA | 20.8 | 26.2 | 27.2 | 0.067 |
| PPI | 13.2 | 18.7 | 38.2 | <0.001 |
| Pseudoephedrine | 15.7 | 21.5 | 24.3 | 0.011 |
| OCS | 8.3 | 10.6 | 12.7 | 0.185 |
| ICS | 25.2 | 25.0 | 23.7 | 0.920 |
| Inhaled short-acting beta-agonists | 2.3 | 3.3 | 1.7 | 0.460 |
| Inhaled long-acting beta-agonists | 20.1 | 19.5 | 19.1 | 0.943 |
| Inhaled antimuscarinics | 4.6 | 4.1 | 5.2 | 0.808 |
| Use of diagnostic tests, % | ||||
| Pulmonary function test | 53.8 | 73.2 | 80.9 | <0.001 |
| Chest CT | 19.4 | 27.6 | 26.0 | 0.005 |
| Blood cell count | 26.8 | 27.8 | 27.2 | 0.938 |
| Induced sputum | 15.7 | 21.9 | 17.3 | 0.032 |
| FeNO | 5.6 | 19.1 | 23.1 | <0.001 |
| Nasopharyngoscopy | 33.2 | 53.4 | 55.5 | <0.001 |
| Laryngoscopy | 31.0 | 51.9 | 53.2 | <0.001 |
| Methacholine challenge | 23.1 | 45.0 | 53.2 | <0.001 |
P value was determined by likelihood ratio chi-square test. H1RA, H1-histamine receptor antihistamines; LTRA, leukotriene receptor antagonists; PPI, proton pump inhibitors; OCS, oral corticosteroids; ICS, inhaled corticosteroids; CT, computed tomography; FeNO, fractional exhaled nitric oxide.
Codeine treatment responses and safety documentation
Based on the medical record reviews, we evaluated the changes of cough status after codeine prescription. Documentation of “improved cough” was found in 40.1% of codeine-prescribed patients and ‘not improved’ in 21.2% within 1–4 weeks of codeine prescription; however, 38.7% was ‘unclear’.
Side effects were documented in 52 codeine-treated patients (7.8%). The most commonly noted side effects were drowsiness (n=16), followed by dry mouth (n=9), constipation (n=9), dizziness (n=6), and gastrointestinal discomfort (n=6).
Discussion
The present study analyzed codeine prescription patterns and explored the treatment response in patients with chronic cough in a real-world setting, using routinely collected institutional EHRs. We found that codeine was prescribed frequently (at least once to 54.0% and for >8 weeks to 14.0% of all patients) and chronically (for >8 weeks to 26.0% of codeine-treated patients and for >200 days in the upper fifth percentile). Several patient factors were significantly associated with longer codeine prescriptions, such as age, cough duration, smoking status, or throat abnormal sensation. Codeine prescription was positively associated with healthcare utilization, such as other cough-related medications, diagnostic tests, or outpatient visits. However, documentation on the effectiveness and safety was lacking in many cases (38.7% and 92.2%, respectively), which indicates the limited utility of current routinely collected data in evaluating treatment responses.
The primary implication of the present analysis is that codeine prescription may be frequent and chronic in real-world practice of patients with chronic cough, despite the lack of robust clinical evidence on the efficacy. In an analysis of a Midwestern academic medical institutional EHRs, US, opioid-containing cough suppressants were prescribed to 22.0% of patients with chronic cough and the odds ratio for at least one prescription was 2.9 for patients with chronic cough (vs. non-chronic cough controls) (22). Similar prescription rates were reported from community-based populations of South Korea and Taiwan (11.9% and 28.2%, respectively) (12), but also a much higher rate reported from specialist clinics (pulmonologists, allergists, otolaryngologists, or gastroenterologists) in Southern California, US (58.9%) (11). The higher codeine prescription rates at specialist clinics (including the present study) may be attributed to healthcare journey or prior insufficient treatment responses of patients before visiting the referral centers (23).
In the present analyses, codeine prescription was significantly associated with several patient factors, such as older age, longer cough duration, less smoking history, and accompanying symptoms (more throat abnormal sensation but less dyspnea). This may reflect specialist clinicians’ perspectives in considering narcotic antitussives. Older age or longer cough duration may indicate a higher probability of longstanding, treatment-refractory or unexplained chronic cough (24,25). Throat abnormal sensation may be associated with central sensitization (20). Older females have heightened cough reflex sensitivity and central cough processing higher than males (26). Because codeine has antitussive effects, presumably by acting on the cough network in the brainstem (20,27), physicians may consider that codeine might be more effective in throat abnormal sensation or without a smoking history or lung parenchymal disease. However, it remains unknown whether these patient characteristics are predictive of codeine treatment response.
Codeine prescription and duration was positively associated with the number of cough-related medications, diagnostic tests, and outpatient visits. Our further analyses were limited by the lack of effectiveness documentation in many cases, but chronic cough patients in needs for codeine-containing antitussives may represent a group of patients with large unmet clinical needs for improved treatment. In our clinical experience, codeine prescription is inevitable in many cases, because there are presently no better alternative antitussive drugs in terms of perceived effectiveness. In the present analysis, the prescription rate of cough neuromodulators, such as amitriptyline or gabapentin, was less than 10% of codeine-treated patients.
We explored codeine treatment responses by manual reviews of routinely collected EHRs but could not identify documentation on the treatment responses in 38.7% of codeine-prescribed patients. Cough was documented as ‘improved’ in 40.1% of the patients, but without use of cough-specific patient-reported outcomes (PROs), it was difficult to properly interpret treatment responses. Given the caveats of observational studies, the rate of ‘improved’ patients is likely to have included those with spontaneous improvements or placebo effects. Conversely, patients with ‘not improved’ cough might be analyzed as a group of interest, but such analyses were limited due to the lack of documentation in 38.7% of codeine-treated cases in this database. Among the patients who were prescribed codeine for more than 8 weeks (26.0%, 173/666), ‘not improved’ and ‘improved’ were mixed. ‘Not improved’ were 28.3% (49/173) among patients who were prescribed for more than 8 weeks, and suppose that the codeine prescription might have been inevitable as there was no alternative drug or that physicians may not have been well aware of typically rapid codeine treatment responses.
The mean 1-year cumulative dose was 1,857.7±3,642.7 mg/year and the mean number of days with codeine prescription was 51.4±66.8 days. This result means that most patients do not take codeine regularly, but adjust it according to symptoms or use it in the beginning and then taper it. Safety is another issue for discussion. Codeine is a relatively weak opiate, and to our knowledge, no major safety problem has been raised regarding the abuse or dependence in adults with chronic cough. However, according to drug treatment monitoring data (28), the risk of codeine misuse or addiction should not be dismissed, although the pharmacovigilance data was not specific to antitussive use. As shown in our analysis, safety data may not be well documented in routine clinical practice. Adverse reaction was documented in 7.8% only. Lack of documentation is not indicative of the absence of safety issue. Education of clinicians is required in the prescription and monitoring of narcotic antitussives.
This study was retrospective and has major limitations that should be further considered. First, our analyses were based on prescription records, which may not correspond to the actual use of codeine. Second, the study was a tertiary institutional database analysis, and has limited external validity. However, the codeine prescription rate was comparable to that reported from a study of specialist clinics, Southern California, US (58.9%) (11). Finally, treatment response was not documented or unclear in 38.7%, even though a manual text review was conducted. This indicates that the value of routinely collected data may be limited in evaluating treatment effectiveness in patients with chronic cough, unless certain decision protocols including PROs are integrated. Physicians should be guided to document treatment responses using PROs, which will improve the utility of real-world data.
Conclusions
In conclusion, our study described codeine prescription pattern using a routinely collected tertiary institutional EHRs of patients with chronic cough. Several patient characteristics such as older age, longer cough duration, less smoking history, or more throat abnormal sensation but less dyspnea were associated with codeine prescription and duration. However, codeine effectiveness was not documented in many cases, and it remains unclear whether such patient factors are predictive of better codeine response. Also, despite the frequent prescription, safety data were lacking for most codeine-prescribed patients. Our findings underscore the unmet clinical need for safe and effective non-narcotic, antitussive drugs and also suggest a need for a stewardship program in guiding the appropriate use of codeine in the real world.
Acknowledgments
Funding: This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease for the series “Novel Insights Into Chronic Cough”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1857/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1857/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1857/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1857/coif). The series “Novel Insights Into Chronic Cough” was commissioned by the editorial office without any funding or sponsorship. WJS served as the unpaid Guest Editor of the series and serves as the unpaid editorial board member of Journal of Thoracic Disease. WJS declares academic grants from MSD, consulting fees from MSD, GSK, AstraZeneca, and Novartis, and honoraria from MSD, GSK, AstraZeneca, and Novartis. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Asan Medical Center (IRB No. 2019-0511) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morice AH. Epidemiology of cough. Pulm Pharmacol Ther 2002;15:253-9. [Crossref] [PubMed]
- Cho SH, Lin HC, Ghoshal AG, et al. Respiratory disease in the Asia-Pacific region: Cough as a key symptom. Allergy Asthma Proc 2016;37:131-40. [Crossref] [PubMed]
- An J, Lee JH, Won HK, et al. Cough Presentation and Cough-Related Healthcare Utilization in Tertiary Care: Analysis of Routinely Collected Academic Institutional Database. Lung 2022;200:431-9. [Crossref] [PubMed]
- Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479-81. [Crossref] [PubMed]
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. [Crossref] [PubMed]
- Yu CJ, Song WJ, Kang SH. The disease burden and quality of life of chronic cough patients in South Korea and Taiwan. World Allergy Organ J 2022;15:100681. [Crossref] [PubMed]
- Irwin RS, French CL, Chang AB, et al. Classification of Cough as a Symptom in Adults and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2018;153:196-209. [Crossref] [PubMed]
- Song DJ, Song WJ, Kwon JW, et al. KAAACI Evidence-Based Clinical Practice Guidelines for Chronic Cough in Adults and Children in Korea. Allergy Asthma Immunol Res 2018;10:591-613. [Crossref] [PubMed]
- Gibson P, Wang G, McGarvey L, et al. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest 2016;149:27-44. [Crossref] [PubMed]
- Eddy NB, Friebel H, Hahn KJ, et al. Codeine and its alternates for pain and cough relief. I. Codeine, exclusive of its antitussive action. Bull World Health Organ 1968;38:673-741. [PubMed]
- Zeiger RS, Schatz M, Butler RK, et al. Burden of Specialist-Diagnosed Chronic Cough in Adults. J Allergy Clin Immunol Pract 2020;8:1645-1657.e7. [Crossref] [PubMed]
- Song WJ, Yu CJ, Kang SH. Cough Characteristics and Healthcare Journeys of Chronic Cough Patients in Community-Based Populations in South Korea and Taiwan. Lung 2022;200:725-36. [Crossref] [PubMed]
- Song WJ, Chung KF. Pharmacotherapeutic Options for Chronic Refractory Cough. Expert Opin Pharmacother 2020;21:1345-58. [Crossref] [PubMed]
- Peechakara BV, Gupta M. Codeine. StatPearls. Treasure Island (FL): StatPearls Publishing LLC., 2022.
- Aylward M, Maddock J, Davies DE, et al. Dextromethorphan and codeine: comparison of plasma kinetics and antitussive effects. Eur J Respir Dis 1984;65:283-91. [PubMed]
- Sevelius H, Colmore JP. Objective assessment of antitussive agents in patients with chronic cough. J New Drugs 1966;6:216-23. [Crossref] [PubMed]
- Sevelius H, McCoy JF, Colmore JP. Dose response to codeine in patients with chronic cough. Clin Pharmacol Ther 1971;12:449-55. [Crossref] [PubMed]
- Morice AH, Menon MS, Mulrennan SA, et al. Opiate therapy in chronic cough. Am J Respir Crit Care Med 2007;175:312-5. [Crossref] [PubMed]
- Smith J, Owen E, Earis J, et al. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2006;117:831-5. [Crossref] [PubMed]
- Chung KF, McGarvey L, Song WJ, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022;8:45. [Crossref] [PubMed]
- Eccles R. The Powerful Placebo Effect in Cough: Relevance to Treatment and Clinical Trials. Lung 2020;198:13-21. [Crossref] [PubMed]
- Weiner M, Liu Z, Schelfhout J, et al. Prescriptions of opioid-containing drugs in patients with chronic cough. Chest. 2019;156:A1791-A2. [Crossref]
- Kang SY, Won HK, Lee SM, et al. Impact of Cough and Unmet Needs in Chronic Cough: A Survey of Patients in Korea. Lung 2019;197:635-9. [Crossref] [PubMed]
- Won HK, Kang SY, Kang Y, et al. Cough-Related Laryngeal Sensations and Triggers in Adults With Chronic Cough: Symptom Profile and Impact. Allergy Asthma Immunol Res 2019;11:622-31. [Crossref] [PubMed]
- Kang SY, Song WJ, Won HK, et al. Cough persistence in adults with chronic cough: A 4-year retrospective cohort study. Allergol Int 2020;69:588-93. [Crossref] [PubMed]
- Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J 2014;44:1149-55. [Crossref] [PubMed]
- Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Curr Opin Allergy Clin Immunol 2007;7:32-6. [Crossref] [PubMed]
- Parry CD, Deluca P, Cooper R, et al. Do we have sufficient information to optimally inform regulatory or other policy decisions about medications containing codeine? Addiction 2015;110:1690-1. [Crossref] [PubMed]



