
Establishment and validation of a long-term prognosis prediction model for patients with non-small cell lung cancer
Highlight box
Key findings
• Our model could accurately identify high risk of death within 5 years of surgery in non-small cell lung cancer patients.
What is known and what is new?
• The incidence of non-small cell lung cancer ranks second among malignant tumors, and the mortality rate ranks first.
• Patients with non-small cell lung cancer have a high mortality rate at 5 years after surgery, and our model could accurately identify high risk of death within 5 years of surgery in non-small cell lung cancer patients.
What is the implication, and what should change now?
• Our model could accurately identify high risk of death within 5 years of surgery in non-small cell lung cancer patients. Strengthening the management of high-risk patients may help improve the prognosis of these patients.
Introduction
The incidence of non-small cell lung cancer ranks second among malignant tumors, and the mortality rate ranks first (1). Surgery is the main method for the radical treatment of non-small cell lung cancer, but even in patients with early-stage lung cancer, the postoperative recurrence rate is still as high as 39%, and postoperative recurrence is also the most important risk factor of death in lung cancer patients (2). To date, studies have confirmed that large tumor diameter, stage III, and surgical method are independent risk factors of mortality in lung cancer patients after surgery (3-7). However, it is still difficult to quantify the risk of postoperative death based on risk factors alone. A nomogram is based on multivariate regression analysis, which integrates multiple indicators, and plots the impact of each index on the patient’s prognosis on a graduated line segment so that risk can be assessed more intuitively (8). Studies of patients with small cell lung cancer have shown that a nomogram predictive model could better assess patient prognosis and identify high-risk patients (9,10). The nomogram predictive model has also been shown to be of good value in identifying high-risk patients with poor prognosis among patients with unresectable lung cancer and in patients after total pneumonectomy (11,12). A study based on the Surveillance, Epidemiology, and End Results (SEER) database showed that in patients with stage IA lung cancer, a nomogram also had some predictive value, but the area under the receiver operating characteristic (ROC) curve was only 0.638 [95% confidence interval (CI): 0.629–0.647] (13). There is currently a lack of research on prediction models for long-term prognosis after surgery in patients with non-small cell lung cancer, and thus we designed the present study. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-381/rc).
Methods
General information
The data of 277 non-small cell lung cancer patients who underwent radical lung cancer resection at Shanghai Fengxian District Central Hospital between January 2016 and December 2017 were retrospectively collected. The patients, who were followed up for 5 years, were divided into a deceased group (n=127) and survival group (n=150) according to whether the patients had died 5 years after surgery or not. The inclusion criteria were: (I) non-small cell lung cancer (intraoperative pathological diagnosis); (II) surgical treatment at our hospital; (III) age ≥18 years old; and (IV) complete clinical data. The exclusion criteria were: (I) combined with other malignant tumors; (II) recurrence of lung cancer after surgery; (III) distant metastases; (IV) small cell lung cancer; (V) did not receive standard treatment; and (VI) loss follow-up. This retrospective study was conducted in accordance with the 2013 edition of the Declaration of Helsinki and approved by the Ethics Committee of Shanghai Fengxian District Central Hospital (No. c202100182). Informed consent was waived in this retrospective study.
Treatment method
In accordance with guidelines for the treatment of lung cancer (14), patients completed preoperative examinations upon admission. All patients underwent radical resection of lung cancer after excluding contraindications to surgery. After surgery, chemotherapy, radiotherapy, or other adjuvant therapy was administered according to the pathological results.
Data collection
Age, gender, body mass index, smoking history, chronic obstructive pulmonary diseases, history of alcoholism, hypertension, diabetes, hyperlipidemia, carcinoembryonic antigen (CEA), tumor site, pathological type, tumor node metastasis (TNM) stage, surgical method, peripheral invasion, vascular tumor thrombus, postoperative radiotherapy, postoperative chemotherapy, and tumor-specific mortality at 5 years after surgery were recorded. Postoperative follow-up was conducted via outpatient visits or telephone.
Statistical analysis
SPSS 26.0 (IBM Corp., Armonk, NY, USA) was used to perform data analysis, and P<0.05 indicated that the difference was statistically significant (two-tailed). The measurement data of the two groups are expressed as mean ± standard deviation (SD), and the independent sample t-test was used to analyze differences in the measurement data of the two groups. Count data of the two groups are expressed as n (%), and the chi-square test was used to analyze the difference in the count data between the two groups. Multivariate logistics regression analysis was used to explore the risk factors of death at 5 years after surgery in patients with non-small cell lung cancer. ROC curves were used to analyze the value of different indexes in predicting the death of non-small cell lung cancer patients at 5 years after surgery. R 4.0.3 statistical software was used to establish the prediction model.
Results
Comparison of clinical features between the two groups
A total of 277 patients with non-small cell lung cancer who underwent surgical treatment were included. A flow chart of the patient selection process is shown in Figure 1. There were significant differences in CEA, TNM stage, peripheral invasion, vascular tumor thrombus, postoperative chemotherapy, and postoperative radiotherapy between the two groups (P<0.05, Table 1).
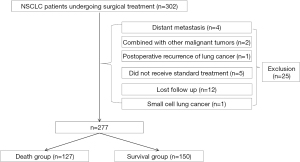
Table 1
| Variables | Deceased group (n=127) | Survival group (n=150) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 59.04±11.06 | 59.19±11.87 | 0.111 | 0.154 |
| Gender | 0.609 | 0.435 | ||
| Male | 82 (64.57) | 90 (60.00) | ||
| Female | 45 (35.43) | 60 (40.00) | ||
| Body mass index (kg/m2) | 25.72±2.61 | 25.64±2.64 | 0.247 | 0.805 |
| History of smoking | 83 (65.35) | 100 (66.67) | 0.053 | 0.818 |
| Chronic obstructive pulmonary diseases | 12(9.45) | 12(8.00) | 0.182 | 0.669 |
| History of alcoholism | 21 (16.54) | 31 (20.67) | 0.770 | 0.380 |
| Hypertension | 12 (9.45) | 11 (7.33) | 0.404 | 0.525 |
| Diabetes | 6 (4.72) | 6 (4.00) | 0.087 | 0.768 |
| Hyperlipidemia | 12 (9.45) | 18 (12.00) | 0.463 | 0.496 |
| CEA (ng/mL) | 226.69±104.60 | 150.75±80.96 | 6.805 | 0.000 |
| CEA >193.5 ng/mL | 80 (62.99) | 43 (28.67) | 32.823 | 0.000 |
| Tumor location | 1.236 | 0.266 | ||
| Left lung | 72 (56.69) | 75 (50.00) | ||
| Right lung | 55 (43.31) | 75 (50.00) | ||
| Type of pathology | 1.889 | 0.389 | ||
| Adenocarcinoma | 89 (70.08) | 116 (77.33) | ||
| Adenosquamous | 31 (24.41) | 28 (18.67) | ||
| Squamous | 7 (5.51) | 6 (4.00) | ||
| TNM staging | 46.316 | 0.000 | ||
| Stage I or II | 62 (48.82) | 130 (86.67) | ||
| Stage III | 65 (51.18) | 20 (13.33) | ||
| Surgical methods | 0.728 | 0.394 | ||
| Minimally invasive surgery | 115 (90.55) | 140 (93.33) | ||
| Open surgery | 12 (9.45) | 10 (6.67) | ||
| Peripheral invasion | 23.575 | 0.000 | ||
| Yes | 23 (18.11) | 2 (1.33) | ||
| No | 104 (81.89) | 148 (98.67) | ||
| Vascular tumor thrombus | 22.101 | 0.000 | ||
| Yes | 29 (22.83) | 6 (4.00) | ||
| No | 98 (77.17) | 144 (96.00) | ||
| Postoperative chemotherapy | 97.341 | 0.000 | ||
| Yes | 123 (96.85) | 61 (40.67) | ||
| No | 4 (3.15) | 89 (59.33) | ||
| Postoperative radiotherapy | 23.440 | 0.000 | ||
| Yes | 49 (38.58) | 20 (13.33) | ||
| No | 78 (61.42) | 130 (86.67) |
Data are presented as mean ± SD or n (%). CEA, carcinoembryonic antigen; TNM, tumor node metastasis; SD, standard deviation.
Predictive value of CEA on tumor-specific death at 5 years after surgery in patients with non-small cell lung cancer
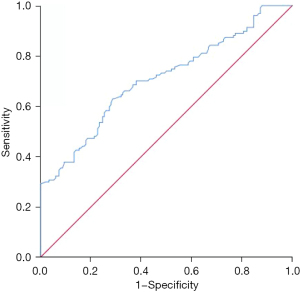
CEA was valuable for predicting tumor-specific death in patients with non-small cell lung cancer at 5 years after surgery, with an area under the curve of 0.707 (95% CI: 0.645–0.769, P=0.000). The best diagnostic cut-off was 193.5 ng/mL, and the sensitivity and specificity were 0.630 and 0.713, respectively (Figure 2).
Risk factors for postoperative tumor-specific death in patients with non-small cell lung cancer
Multivariate logistics regression analysis showed that CEA >193.5 ng/mL, stage III, peripheral invasion, and vascular tumor thrombus were independent risk factors of tumor-specific death at 5 years after surgery in patients with non-small cell lung cancer (P<0.05, Table 2).
Table 2
| Variables | B value | Standard error | Wald value | P value | Relative risk (95% CI) |
|---|---|---|---|---|---|
| CEA >193.5 ng/mL | 1.338 | 0.292 | 21.045 | 0.000 | 3.810 (2.152–6.748) |
| Stage III | 1.664 | 0.329 | 25.635 | 0.000 | 5.279 (2.772–10.052) |
| Peripheral invasion | 2.042 | 0.797 | 6.575 | 0.010 | 7.709 (1.618–36.732) |
| Vascular tumor thrombus | 1.294 | 0.550 | 5.540 | 0.019 | 3.646 (1.242–10.708) |
| Constant | −11.143 | 1.890 | 34.777 | 0.000 | 0.000 |
CI, confidence interval; CEA, carcinoembryonic antigen.
Establishment and validation of tumor-specific death prediction model in patients with non-small cell lung cancer 5 years after surgery
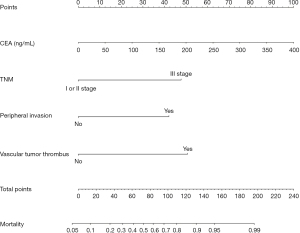
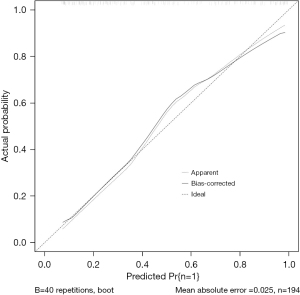
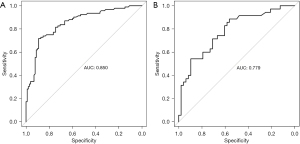
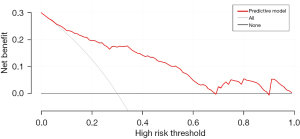
R 4.0.3 statistical software was used to randomly divide the dataset into a training set and validation set. The sample size of the training set was 194 and the sample size of the validation set was 83. The area under the ROC curve was 0.850 (95% CI: 0.796–0.905) in the training set, and it was 0.779 (95% CI: 0.678–0.880) in the validation set. In the validation set, the model was assessed using the Hosmer-Lemeshow goodness-of-fit test, with a chi-square value of 9.270 and a P value of 0.320 (Figures 3-6).
Discussion
We designed this study to more accurately identify people at high risk of tumor-specific death at 5 years after surgery in patients with non-small cell lung cancer, and in the present study, we hoped to predict the risk of death for patients at the time of admission, so we did not include factors related to postoperative treatment. In the present study, multivariate logistics regression analysis showed that CEA >193.5 ng/mL, stage III lung cancer, peritumor invasion, and vascular tumor thrombus were independent risk factors of tumor-specific death after surgery in patients with non-small cell lung cancer (P<0.05). R 4.0.3 statistical software was used to randomly divide the dataset into a training set and validation set. The sample size of the training set was 194, and the sample size of the validation set was 83. The area under the ROC curve was 0.850 (95% CI: 0.796–0.905) in the training set, and the area under the curve of the ROC was 0.779 (95% CI: 0.678–0.880) in the validation set. In the validation set, the model was assessed using the Hosmer-Lemeshow goodness-of-fit test, with a chi-square value of 9.270 and a P value of 0.320, indicating that the predictive value and reliability of the model were high.
As a broad-spectrum tumor marker, CEA is not used as a specific indicator for the diagnosis of a malignant tumor. However, in the differential diagnosis and efficacy evaluation of malignant tumors, CEA is still important. Increased CEA in non-small cell lung cancer patients indicates that recurrence and metastasis are more likely to occur, and recent studies have shown that CEA is valuable for predicting prognosis in non-small cell lung cancer patients (15-18). The TNM staging system is used to comprehensively evaluate the stage of lung cancer. As stage III non-small cell lung cancer patients have regional lymph node metastasis, they are more likely to have tumor-specific death (19-21). Lung cancer can invade surrounding tissues, including in the superior vena cava, aorta, pericardium, pleura, and other important tissues of the mediastinum. When peripheral invasion occurs, it indicates that the tumor has potential to spread, leading to postoperative recurrence and metastasis, and eventually death (22). Finally, the formation of vascular tumor thrombus has been confirmed to be closely related to distant metastasis and lymph node metastasis (23,24). Up to now, previous studies have confirmed that increased CEA, high TNM staging, peripheral invasion and vascular tumor thrombus were independent risk factors of death in non-small cell lung cancer, supporting our study (18,19,22,23).
However, the prognosis of patients with non-small cell lung cancer is affected by many factors, so the predictive value of a single biological indicator on prognosis is limited. Therefore, the nomogram predictive model was developed. A study of non-small cell lung cancer patients with stage N3 showed that the nomogram predictive model could predict patient outcomes better than a single biological marker (25). The present study also showed that the established nomogram prediction model had more value than a single biological indicator such as CEA in predicting tumor-specific death at 5 years after surgery in patients with non-small cell lung cancer. Thus, the prediction model may be valuable in identifying the patients as high risk of death.
Limitations
The present study had the limitations characteristic of a retrospective study. Furthermore, the degree of malignancy in lung cancer patients is affected by genes and other factors (such as epidermal growth factor receptor mutations), and the present study failed to explore genes and other related indicators. Finally, the diagnostic model in the present study lacks external validation.
Conclusions
Prognosis and related indicators of different diseases are the focus of current research (26-29). Our model could accurately identify high risk of death within 5 years of surgery in non-small cell lung cancer patients. Enhanced management of these high-risk populations may help improve patient outcomes, but further clinical studies are needed.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of China (No. 81960577).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-381/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-381/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-381/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-381/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was conducted in accordance with the 2013 edition of the Declaration of Helsinki and approved by the Ethics Committee of Shanghai Fengxian District Central Hospital (No. c202100182). Informed consent was waived in this retrospective study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Nomori H, Mori T, Shiraishi A, et al. Long-Term Prognosis After Segmentectomy for cT1 N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1500-6. [Crossref] [PubMed]
- Su P, Zhu Y, Lv H, et al. Nomogram Prediction Model Analysis of Risk Factors for Conversion to Thoracotomy after Thoracoscopic Resection of Lung Cancer and Prognostic Value of Lung Cancer. Comput Math Methods Med 2022;2022:3628335. [Crossref] [PubMed]
- Morishima T, Kuwabara Y, Saito MK, et al. Patterns of staging, treatment, and mortality in gastric, colorectal, and lung cancer among older adults with and without preexisting dementia: a Japanese multicentre cohort study. BMC Cancer 2023;23:67. [Crossref] [PubMed]
- Balbi M, Sabia F, Ledda RE, et al. Automated Coronary Artery Calcium and Quantitative Emphysema in Lung Cancer Screening: Association With Mortality, Lung Cancer Incidence, and Airflow Obstruction. J Thorac Imaging 2023; Epub ahead of print. [Crossref] [PubMed]
- Almberg KS, Halldin CN, Friedman LS, et al. Increased odds of mortality from non-malignant respiratory disease and lung cancer are highest among US coal miners born after 1939. Occup Environ Med 2023;80:121-8. [Crossref] [PubMed]
- He H, He MM, Wang H, et al. In Utero and Childhood/Adolescence Exposure to Tobacco Smoke, Genetic Risk, and Lung Cancer Incidence and Mortality in Adulthood. Am J Respir Crit Care Med 2023;207:173-82. [Crossref] [PubMed]
- Zhou H, Zhang Y, Qiu Z, et al. Nomogram to Predict Cause-Specific Mortality in Patients With Surgically Resected Stage I Non-Small-Cell Lung Cancer: A Competing Risk Analysis. Clin Lung Cancer 2018;19:e195-203. [Crossref] [PubMed]
- Zhong J, Zheng Q, An T, et al. Nomogram to predict cause-specific mortality in extensive-stage small cell lung cancer: A competing risk analysis. Thorac Cancer 2019;10:1788-97. [Crossref] [PubMed]
- Li J, Zheng Q, Zhao X, et al. Nomogram model for predicting cause-specific mortality in patients with stage I small-cell lung cancer: a competing risk analysis. BMC Cancer 2020;20:793. [Crossref] [PubMed]
- Yu X, Gao S, Xue Q, et al. Development of a nomogram for predicting the operative mortality of patients who underwent pneumonectomy for lung cancer: a population-based analysis. Transl Lung Cancer Res 2021;10:381-91. [Crossref] [PubMed]
- Yang Y, Shen C, Shao J, et al. Based on the Development and Verification of a Risk Stratification Nomogram: Predicting the Risk of Lung Cancer-Specific Mortality in Stage IIIA-N2 Unresectable Large Cell Lung Neuroendocrine Cancer Compared With Lung Squamous Cell Cancer and Lung Adenocarcinoma. Front Oncol 2022;12:825598. [Crossref] [PubMed]
- Yang H, Li X, Shi J, et al. A nomogram to predict prognosis in patients undergoing sublobar resection for stage IA non-small-cell lung cancer. Cancer Manag Res 2018;10:6611-26. [Crossref] [PubMed]
- Health Commission Of The People's Republic Of China N. National guidelines for diagnosis and treatment of lung cancer 2022 in China (English version). Chin J Cancer Res 2022;34:176-206. [Crossref]
- Li G, Zhang H, Zhang L, et al. Serum Markers CA125, CA153, and CEA along with Inflammatory Cytokines in the Early Detection of Lung Cancer in High-Risk Populations. Biomed Res Int 2022;2022:1394042. [Crossref] [PubMed]
- Bi H, Yin L, Fang W, et al. Association of CEA, NSE, CYFRA 21-1, SCC-Ag, and ProGRP with Clinicopathological Characteristics and Chemotherapeutic Outcomes of Lung Cancer. Lab Med 2022; Epub ahead of print. [Crossref] [PubMed]
- He L, Chen X, Ding L, et al. Clinical Efficacy of Antianlotinib Combined with Immune Checkpoint Inhibitors in the Treatment of Advanced Non-Small-Cell Lung Cancer and Its Effect on Serum VEGF, CEA, and SCC-Ag. J Oncol 2022;2022:1530875. [Crossref] [PubMed]
- Zheng J, Wang Y, Hu C, et al. Predictive value of early kinetics of ctDNA combined with cfDNA and serum CEA for EGFR-TKI treatment in advanced non-small cell lung cancer. Thorac Cancer 2022;13:3162-73. [Crossref] [PubMed]
- Erdoğu V, Çıtak N, Sezen CB, et al. Comparison of 6th, 7th, and 8th editions of the TNM staging in non-small cell lung cancer patients: Validation of the 8th edition of TNM staging. Turk Gogus Kalp Damar Cerrahisi Derg 2022;30:395-403.
- Solberg S, Nilssen Y, Terje Brustugun O, et al. Concordance between clinical and pathology TNM-staging in lung cancer. Lung Cancer 2022;171:65-9. [Crossref] [PubMed]
- Tan F, Bi N, Zhang H, et al. External validation of the eighth edition of the TNM classification for lung cancer in small cell lung cancer. Lung Cancer 2022;170:98-104.
- Chen Q, Shao J, Xue T, et al. Intratumoral and peritumoral radiomics nomograms for the preoperative prediction of lymphovascular invasion and overall survival in non-small cell lung cancer. Eur Radiol 2023;33:947-58. [Crossref] [PubMed]
- Henglian L, Jiajun W, Caixia W, et al. Analysis of related risk factors of lung metastasis after laparoscopic radical hysterectomy of cervical cancer. Medicine (Baltimore) 2021;100:e24480. [Crossref] [PubMed]
- Qiao XJ, Gu Y, Du H, et al. Co-expression of CD24 and Hsp70 as a prognostic biomarker for lung cancer. Neoplasma 2021;68:1023-32. [Crossref] [PubMed]
- Han C, Wu Y, Sun X, et al. Outcome of Non-small Cell Lung Cancer Patients With N3 Stage: Survival Analysis of Propensity Score Matching With a Validated Predictive Nomogram. Front Surg 2021;8:666332. [Crossref] [PubMed]
- Chen H, Meng X, Hao X, et al. Correlation Analysis of Pathological Features and Axillary Lymph Node Metastasis in Patients with Invasive Breast Cancer. J Immunol Res 2022;2022:7150304. [Crossref] [PubMed]
- Qiu Y, Chen H, Dai Y, et al. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients' Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J 2022;2022:6705052. [Crossref] [PubMed]
- Qiu Y, Chen Y, Zhu L, et al. Differences of Clinicopathological Features between Metaplastic Breast Carcinoma and Nonspecific Invasive Breast Carcinoma and Prognostic Profile of Metaplastic Breast Carcinoma. Breast J 2022;2022:2500594. [Crossref] [PubMed]
- Chen Y, Si H, Bao B, et al. Integrated analysis of intestinal microbiota and host gene expression in colorectal cancer patients. J Med Microbiol 2022; [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


