
Hypothyroidism induced by immune checkpoint inhibitors combined with antiangiogenic agents is associated with higher body mass index
Highlight box
Key findings
• High body mass index (BMI) is associated with hypothyroidism induced by immune checkpoint inhibitors (ICIs) plus antiangiogenic agents.
What is known and what is new?
• Hypothyroidism is often observed in patients receiving immune checkpoint blockade, and antiangiogenic agents are also known to be associated with hypothyroidism. However, the incidence rate and risk factors of hypothyroidism caused by ICIs combined with antiangiogenic therapy are still not well understood.
• A 28.5% incidence rate of any grade hypothyroidism was reported in this cohort. The logistic analysis demonstrated that a higher BMI was significantly associated with an increased risk of hypothyroidism induced by ICIs plus antiangiogenic agents.
What is the implication, and what should change now?
• Our findings demonstrate that although the risk of hypothyroidism in patients receiving a combination of ICIs and antiangiogenic agents is manageable, clinicians should perform regular thyroid function monitoring in high-risk groups during the combination therapy.
Introduction
Immune checkpoint inhibitors (ICIs) are often used to treat non-small cell lung cancer (NSCLC); however, many patients do not benefit from these therapies. Studies have shown that the combination of ICIs and antiangiogenic medicines is effective and safe in the treatment of advanced NSCLC (1,2). The IMPOWER 150 study demonstrated that atezolizumab combined with bevacizumab prolonged the progression-free survival and overall survival of patients with advanced NSCLC (3,4). The overall effectiveness and safety of pembrolizumab coupled with ramucirumab for the treatment of patients with advanced NSCLC were good, as reported in the JVDF trial (5). In the phase II Passion study, camrelizumab combined with apatinib demonstrated good efficacy and safety in the treatment of advanced NSCLC (6). However, both ICIs and antiangiogenic agents cause a number of adverse effects, and the toxicity spectrum can be more complex when used together than when used alone.
Thyroid dysfunction, such as hypothyroidism, hyperthyroidism, and thyroiditis, has been documented in up to 50% of patients treated with ICI-based combination therapies in several trials (6-9). Notably, a previous study (10) reported that combination immunotherapy was associated with a high estimated incidence of high thyroid dysfunction frequencies, ranging from 8.0% to 16.4%, which is significantly higher than that of monotherapy with programmed cell death-1 (PD-1), programmed cell death ligand 1 (PD-L1) inhibitors, or drugs targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). In addition, studies have also demonstrated that patients who receive antiangiogenic monoclonal antibodies and small molecule anti-vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors (TKIs) have a significantly increased risk of hypothyroidism (11-13).
The endocrine toxicities caused by immune therapy combined with antiangiogenic agents have rarely been studied. A previous systematic review (14) showed that the total incidence of severe adverse events (AEs) associated with ICIs plus antiangiogenic monoclonal antibodies (mAbs) is lower than that of ICIs plus TKIs. The most commonly reported AEs of immune therapy combined with antiangiogenic agents included thrombocytopenia and fatigue, while nausea, vomiting, and immune pneumonia were relatively rare. Another retrospective cohort study including three clinical trials in patients with stage III/IV melanoma treated with anti-PD-1 and antiangiogenic therapy showed that the treatment-related AEs (trAEs) mainly affected the liver, endocrine, skin, and gastrointestinal tract, followed by myelosuppression, renal insufficiency, and dyslipidemia (15). Overall, the trAE spectra are similar to those found in ICIs or antiangiogenic treatment monotherapy and are not severe.
Recent studies investigated the potential risk factors of thyroid immune-related AEs (irAEs). Specifically, the presence of baseline thyroid autoantibodies and the number of ICI treatment cycles were associated with an increased risk (16-18). Emerging evidence has suggested that the increased irAEs in cancer patients receiving ICIs are linked to the obese and overweight body mass index (BMI) categories (19). Obesity is a low-grade inflammatory metabolic condition associated with many coexisting diseases, including diabetes, cardiovascular disease, and cancer (20-25).
It is speculated that the combined use of ICIs and antiangiogenic agents may increase the risk and complexity of hypothyroidism. Based on this hypothesis, we intend to evaluate the incidence of hypothyroidism in NSCLC patients receiving ICIs combined with antiangiogenic agents, evaluate the risk factors of hypothyroidism caused by ICIs combined with antiangiogenic therapy, and explore the relationship between BMI and treatment-related hypothyroidism. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-306/rc).
Methods
Study design and participants
We conducted a retrospective study using data from the Tianjin Medical University Cancer Institute & Hospital. This study included patients with advanced NSCLC aged 18 or older who received PD-1 inhibitors (nivolumab, pembrolizumab, tislelizumab, camrelizumab, sintilimab) or PD-L1 inhibitors (durvalumab, atezolizumab) combined with antiangiogenic therapy (bevacizumab, anlotinib, apatinib) in our hospital from July 1, 2019, to December 31, 2021. The patients’ complete medical data were recorded in electronic medical records, including medical diagnoses, laboratory tests, prescription drugs, follow-up information, etc. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional Ethics Committee of Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Tianjin, China (approval number: bc2022098). Due to the anonymity of patient records, the individual consent for this retrospective analysis was waived.
Baseline assessments were defined as those taken within 2 weeks before the administration of combined therapy. Patient characteristics and clinical data included age, gender, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), smoking status, histology, comorbidities, combination therapy with or without chemotherapy, PD-L1 expression, ICI drug target, treatment line, height, and weight. The baseline laboratory test variables, including white blood cell count, neutrophil count, lymphocyte count, platelet count, monocyte count, eosinophil count, hemoglobin, and albumin, were analyzed as continuous variables. In addition, thyroid function tests (TFTs) within 1 month before the treatment start date, including records of thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3), were collected. Specifically, a TFT was required before each treatment cycle, and at least five TFTs were recorded during the treatment. The related symptoms of abnormal thyroid function were also collected. Patients with a history of thyroid dysfunction prior to treatment, including a previously diagnosed thyroid dysfunction listed in the medical history, who had previously received antithyroid drugs or levothyroxine treatment, or if the TFT was abnormal at baseline, were excluded. We also excluded cases with missing values, including 17 cases without baseline height or weight data, 55 patients with missing TFTs during the follow-up, and 14 patients for whom we were unable to evaluate the response. Tumor responses were evaluated using the Response Evaluation Criteria for Solid Tumors version 1.1. The median follow-up time was 10.9 months. The study flow diagram is shown in Figure 1.
BMI assessment
BMI was calculated using patients’ height and weight, which was measured within 2 weeks before combination treatment initiation, and was analyzed as both a continuous and a categorized variable. The patients were categorized into three categories based on their baseline BMI, according to the World Health Organization (WHO) Regional Committee for the Western Pacific standard (26): low-normal BMI <23 kg/m2; overweight 23–24.9 kg/m2; obese >25 kg/m2.
Assessment of outcomes
Hypothyroidism outcomes were defined as follows: (I) any hypothyroidism (overt and subclinical) was defined as a TSH level greater than the laboratory-specific reference range, regardless of the fT4 and fT3 values during follow-up; (II) overt hypothyroidism was defined as decreased fT4 levels and/or decreased fT3 levels, or elevated TSH levels of 10 mIU/L or higher; (III) subclinical hypothyroidism was defined as TSH greater than the laboratory-specific reference range but <10 mIU/L, and without a corresponding fT4 or fT3 exception. The multiple definitions of the study outcomes were used to evaluate the robustness of the results.
Statistical analysis
Continuous variables were described as the mean ± standard deviation. Comparisons between two outcome groups were performed using either the Student’s t-test, Wilcoxon rank sum test, Chi-square test, or Fisher’s exact test, as appropriate. A logistic regression model was employed in the univariate and multivariate analysis to identify risk factors for any grade hypothyroidism or overt hypothyroidism, and multivariate logistic analysis was conducted to reduce the effects of potential confounders. Backward stepwise elimination based on the Wald test was used to reduce the number of predictors in the multivariable logistic regression models, as the sample size was comparatively small and the prevalence of outcome was relatively low, and a liberal P value of 0.10 was used. Two variables were introduced in a multivariable logistic regression model predicting hypothyroidism, and one predictor was introduced in the model predicting overt hypothyroidism. Therefore, the sample size of this study was considered suitable to obtain accurate results. All P values presented were two-sided. P<0.05 was deemed statistically significant. R software was used for all statistical analyses (version 4.2.1, R Foundation, Vienna, Austria).
Results
Study population characteristics
A total of 137 patients were enrolled in this study. The clinical characteristics of all included patients are shown in Table 1. The average age of the patients was 61.12±9.41 years old, and the average BMI of the patients was 23.90±3.31 kg/m2. This cohort was dominated by male patients (82.5%) and patients with ECOG-PS of 0 or 1 (86.1%). The majority of patients (71.5%) were current or former smokers. Squamous cell carcinoma histology accounted for 35.8% of the subjects. The combination of ICIs, antiangiogenesis agents, and chemotherapy was administered to 42.3% of patients in our cohort. Most of the patients (80.3%) received the combination regimen as a first- or second-line therapy. Among this cohort, the objective response rate was 35.8%.
Table 1
| Variables | Total, n=137 |
|---|---|
| Age (years) | 61.12 (9.41) |
| Gender | |
| Female | 24 [17.5] |
| Male | 113 [82.5] |
| ECOG-PS | |
| 0 | 51 [37.2] |
| 1 | 67 [48.9] |
| 2 | 19 [13.9] |
| Smoking history | |
| Never | 39 [28.5] |
| Current or former | 98 [71.5] |
| Histology | |
| Non-squamous | 88 [64.2] |
| Squamous | 49 [35.8] |
| Diabetes, n (%) | |
| Without | 118 [86.1] |
| With | 19 [13.9] |
| Combined chemotherapy | |
| Without | 79 [57.7] |
| With | 58 [42.3] |
| PD-L1 expression | |
| <1% | 27 [19.7] |
| ≥1% | 35 [25.6] |
| Unknown | 75 [54.7] |
| ICI drug target | |
| PD-L1 | 10 [7.3] |
| PD-1 | 127 [92.7] |
| Line of ICI therapy | |
| 1 | 44 [32.1] |
| 2 | 66 [48.2] |
| ≥3 | 27 [19.7] |
| Best tumor response | |
| PR | 49 [35.8] |
| SD | 69 [50.3] |
| PD | 19 [13.9] |
| BMI (kg/m2) | 23.90 (3.31) |
| BMI | |
| Low-normal | 56 [40.9] |
| Overweight | 31 [22.6] |
| Obese | 50 [36.5] |
| WBC (109/L) | 7.00 (2.65) |
| NEU (109/L) | 5.82 (8.06) |
| LYM (109/L) | 1.86 (3.22) |
| PLT (109/L) | 254.39 (97.45) |
| MONO (109/L) | 0.61 (0.28) |
| AEC (109/L) | 0.20 (0.31) |
| Hb (g/L) | 129.45 (20.65) |
| ALB (g/L) | 94.55 (28.60) |
Values are expressed as the mean (standard deviation) or n [%]. ECOG-PS, the Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death protein ligand-1; PD-1, programmed cell death protein-1; ICI, immune checkpoint inhibitor; PR, partial response; SD, stable disease; PD, progressive disease; BMI, body mass index; WBC, white blood cell count; NEU, neutrophil count; LYM, lymphocyte count; PLT, platelet count; MONO, monocytes count; AEC, absolute eosinophil count; Hb, hemoglobin; ALB, albumin.
Comparisons of characteristics in patients with and without hypothyroidism
As shown in Table 2, 39 (28.5%) of the 137 patients developed hypothyroidism. Among them, 20 cases (14.6%) were overt hypothyroidism and 19 cases (13.9%) were subclinical hypothyroidism. Of the 39 patients with hypothyroidism, 17 received levothyroxine replacement therapy, and only one of them required steroids and discontinuation of therapy.
Table 2
| Variables | Any hypothyroidism | Subclinical hypothyroidism | Overt hypothyroidism | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| + (n=39) | − (n=98) | P value | + (n=19) | − (n=118) | P value | + (n=20) | − (n=117) | P value | |||
| Age (years) | 64.59 (7.19) | 59.74 (9.85) | 0.006* | 66.63 (6.65) | 60.24 (9.51) | 0.006* | 62.65 (7.31) | 60.86 (9.72) | 0.435 | ||
| Gender | 0.869 | 1.000 | 0.998 | ||||||||
| Female | 6 [15.4] | 18 [18.4] | 3 [15.8] | 21 [17.8] | 3 [15.0] | 21 [17.9] | |||||
| Male | 33 [84.6] | 80 [81.6] | 16 [84.2] | 97 [82.2] | 17 [85.0] | 96 [82.1] | |||||
| ECOG-PS | 0.645 | 0.694 | 0.286 | ||||||||
| 0 | 13 [33.3] | 38 [38.8] | 6 [31.6] | 45 [38.1] | 7 [35.0] | 44 [37.6] | |||||
| 1 | 19 [48.7] | 48 [49.0] | 11 [57.9] | 56 [47.5] | 8 [40.0] | 59 [50.4] | |||||
| 2 | 7 [18.0] | 12 [12.2] | 2 [10.5] | 17 [14.4] | 5 [25.0] | 14 [12.0] | |||||
| Smoking history | 0.131 | 0.619 | 0.240 | ||||||||
| Never | 7 [17.9] | 32 [32.7] | 4 [21.1] | 35 [29.7] | 3 [15.0] | 36 [30.8] | |||||
| Current or former | 32 [82.1] | 66 [67.3] | 15 [78.9] | 83 [70.3] | 17 [85.0] | 81 [69.2] | |||||
| Histology | 0.859 | 0.879 | 1.000 | ||||||||
| Non-squamous | 26 [66.7] | 62 [63.3] | 13 [68.4] | 75 [63.6] | 13 [65.0] | 75 [64.1] | |||||
| Squamous | 13 [33.3] | 36 [36.7] | 6 [31.6] | 43 [36.4] | 7 [35.0] | 42 [35.9] | |||||
| Diabetes | 1.000 | 0.536 | 0.373 | ||||||||
| Without | 34 [87.2] | 84 [85.7] | 15 [78.9] | 103 [87.3] | 19 [95.0] | 99 [84.6] | |||||
| With | 5 [12.8] | 14 [14.3] | 4 [21.1] | 15 [12.7] | 1 [5.0] | 18 [15.4] | |||||
| Combined chemotherapy | 0.446 | 1.000 | 0.319 | ||||||||
| Without | 20 [51.3] | 59 [60.2] | 11 [57.9] | 68 [57.6] | 9 [45.0] | 70 [59.8] | |||||
| With | 19 [48.7] | 39 [39.8] | 8 [42.1] | 50 [42.4] | 11 [55.0] | 47 [40.2] | |||||
| PD-L1 expression | 0.916 | 0.711 | 0.825 | ||||||||
| <1% | 8 [20.5] | 19 [19.4] | 5 [26.3] | 22 [18.6] | 3 [15.0] | 24 [20.5] | |||||
| ≥1% | 9 [23.1] | 26 [26.5] | 4 [21.1] | 31 [26.3] | 5 [25.0] | 30 [25.6] | |||||
| Unknown | 22 [56.4] | 53 [54.1] | 10 [52.6] | 65 [55.1] | 12 [60.0] | 63 [53.9] | |||||
| ICI drug target | 0.229 | 0.914 | 0.333 | ||||||||
| PD-L1 | 5 [12.8] | 5 [5.1] | 2 [10.5] | 8 [6.8] | 3 [15.0] | 7 [6.0] | |||||
| PD-1 | 34 [87.2] | 93 [94.9] | 17 [89.5] | 110 [93.2] | 17 [85.0] | 110 [94.0] | |||||
| Line of ICI therapy | 0.215 | 0.502 | 0.452 | ||||||||
| 1 | 14 [35.9] | 30 [30.6] | 6 [31.6] | 38 [32.2] | 8 [40.0] | 36 [30.8] | |||||
| 2 | 21 [53.8] | 45 [45.9] | 11 [57.9] | 55 [46.6] | 10 [50.0] | 56 [47.8] | |||||
| ≥3 | 4 [10.3] | 23 [23.5] | 2 [10.5] | 25 [21.2] | 2 [10.0] | 25 [21.4] | |||||
| Best tumor response, n (%) | 0.876 | 0.902 | 0.844 | ||||||||
| PR | 13 [33.3] | 36 [36.7] | 7 [36.9] | 42 [35.6] | 6 [30.0] | 43 [36.7] | |||||
| SD | 21 [53.9] | 48 [49.0] | 10 [52.6] | 59 [50.0] | 11 [55.0] | 58 [49.6] | |||||
| PD | 5 [12.8] | 14 [14.3] | 2 [10.5] | 17 [14.4] | 3 [15.0] | 16 [13.7] | |||||
| BMI (kg/m2) | 25.46 (3.25) | 23.28 (3.14) | <0.001* | 25.60 (3.77) | 23.63 (3.16) | 0.015* | 25.33 (2.77) | 23.65 (3.34) | 0.035* | ||
| BMI | <0.001* | 0.032* | 0.016* | ||||||||
| Low-normal | 8 [20.5] | 48 [49.0] | 4 [21.0] | 52 [44.1] | 4 [20.0] | 52 [44.4] | |||||
| Overweight | 6 [15.4] | 25 [25.5] | 3 [15.8] | 28 [23.7] | 3 [15.0] | 28 [24.0] | |||||
| Obese | 25 [64.1] | 25 [25.5] | 12 [63.2] | 38 [32.2] | 13 [65.0] | 37 [31.6] | |||||
| WBC (109/L) | 7.14 (2.18) | 6.95 (2.82) | 0.716 | 7.20 (2.55) | 6.97 (2.67) | 0.734 | 7.08 (1.82) | 6.99 (2.77) | 0.895 | ||
| NEU (109/L) | 4.86 (1.77) | 6.21 (9.45) | 0.379 | 5.16 (2.01) | 5.93 (8.65) | 0.702 | 4.57 (1.49) | 6.04 (8.69) | 0.454 | ||
| LYM (109/L) | 1.50 (0.68) | 2.00 (3.78) | 0.413 | 1.33 (0.65) | 1.95 (3.45) | 0.439 | 1.67 (0.69) | 1.89 (3.47) | 0.772 | ||
| PLT (109/L) | 254.82 (87.27) | 254.22 (101.64) | 0.974 | 260.05 (97.47) | 253.48 (97.83) | 0.786 | 249.85 (78.60) | 255.17 (100.58) | 0.823 | ||
| MONO (109/L) | 0.59 (0.20) | 0.62 (0.30) | 0.661 | 0.59 (0.23) | 0.61 (0.28) | 0.735 | 0.60 (0.18) | 0.61 (0.29) | 0.820 | ||
| AEC (109/L) | 0.15 (0.12) | 0.22 (0.36) | 0.269 | 0.14 (0.11) | 0.21 (0.33) | 0.337 | 0.17 (0.13) | 0.20 (0.33) | 0.639 | ||
| Hb (g/L) | 128.64 (18.21) | 129.78 (21.62) | 0.773 | 127.16 (22.84) | 129.82 (20.36) | 0.604 | 130.05 (12.83) | 129.35 (21.74) | 0.889 | ||
| ALB (g/L) | 41.53 (5.05) | 40.81 (4.83) | 0.440 | 42.98 (6.19) | 40.70 (4.60) | 0.059 | 40.16 (3.25) | 41.17 (5.11) | 0.395 | ||
Values are expressed as the mean (standard deviation) or n [%]. *, significant P values <0.05. ECOG-PS, the Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death protein ligand-1; PD-1, programmed cell death protein-1; ICI, immune checkpoint inhibitor; PR, partial response; SD, stable disease; PD, progressive disease; BMI, body mass index; WBC, white blood cell; NEU, neutrophil; LYM, lymphocyte; PLT, platelet; MONO, monocytes; AEC, absolute eosinophil count; Hb, hemoglobin; ALB, albumin.
The average BMI of patients with hypothyroidism was significantly higher than that of patients with normal thyroid function (25.46±3.25 vs. 23.28±3.14, P<0.001), and their age was also significantly older than that of patients with normal thyroid function (64.59±7.19 vs. 59.74±9.85, P=0.006). The mean baseline BMI was also significantly higher in patients who developed overt hypothyroidism than that of the other patients (25.33±2.77 vs. 23.65±3.34, P=0.035), while the distribution of their baseline characteristics other than BMI was similar to that of the other patients. Moreover, there was no significant difference in the laboratory examination results between patients with and without hypothyroidism or in the proportion of tumor response between the outcome groups.
Logistic regression analyses of the risk of hypothyroidism
In the univariate logistic regression analyses, we analyzed BMI as a continuous variable and found that the risk of hypothyroidism continued to increase with the increase in BMI [odds ratio (OR) 1.24, 95% confidence interval (CI): 1.10–1.42; P<0.001, Table 3 and Figure 2A]. BMI was also a significant risk factor for overt hypothyroidism (OR 1.17, 95% CI: 1.01–1.38, P=0.039, Table 4 and Figure 2B). The multivariate logistic regression analysis showed that baseline BMI (OR 1.36, 95% CI: 1.16–1.61, P<0.001) and age (OR 1.08, 95% CI: 1.02–1.14, P=0.006, Table 3) were independent predictors of hypothyroidism. Furthermore, the multivariate analysis also showed that only BMI was an independent risk factor for overt hypothyroidism (OR =1.23, 95% CI: 1.03–1.52, P=0.029, Table 4).
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (continuous) | 1.07 (1.02–1.12) | 0.008* | 1.08 (1.02–1.14) | 0.006* | |
| Gender (female/male) | 1.24 (0.47–3.66) | 0.679 | – | – | |
| ECOG-PS (0–1/2) | 1.57 (0.54–4.26) | 0.386 | – | – | |
| Smoking (never/current or former) | 2.22 (0.92–5.95) | 0.090 | – | – | |
| Histology (non-squamous/squamous) | 0.86 (0.39–1.86) | 0.708 | – | – | |
| Diabetes (without/with) | 0.88 (0.27–2.51) | 0.823 | – | – | |
| Combined chemotherapy (without/with) | 1.44 (0.68–3.04) | 0.341 | – | – | |
| ICI drug target (PD-L1/PD-1) | 0.37 (0.10–1.39) | 0.129 | – | – | |
| Line of ICI therapy (continuous) | 0.69 (0.40–1.17) | 0.171 | – | – | |
| Best tumor response (PR + SD/PD) | 1.19 (0.39–3.28) | 0.746 | – | – | |
| BMI (continuous) | 1.24 (1.10–1.42) | <0.001* | 1.36 (1.16–1.61) | <0.001* | |
| WBC (continuous) | 1.03 (0.89–1.18) | 0.714 | – | – | |
| NEU (continuous) | 0.97 (0.86–1.02) | 0.418 | – | – | |
| LYM (continuous) | 0.90 (0.57–1.06) | 0.489 | – | – | |
| PLT (continuous) | 1.00 (1.00–1.00) | 0.974 | – | – | |
| MONO (continuous) | 0.73 (0.17–2.82) | 0.658 | – | – | |
| AEC (continuous) | 0.38 (0.04–1.60) | 0.286 | – | – | |
| Hb (continuous) | 1.00 (0.98–1.02) | 0.771 | – | – | |
| ALB (continuous) | 1.03 (0.96–1.12) | 0.437 | – | – | |
*, significant P values <0.05. ECOG-PS, the Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death protein ligand-1; PD-1, programmed cell death protein-1; ICI, immune checkpoint inhibitor; PR, partial response; SD, stable disease; PD, progressive disease; BMI, body mass index; WBC, white blood cell; NEU, neutrophil; LYM, lymphocyte; PLT, platelet; MONO, monocytes; AEC, absolute eosinophil count; Hb, hemoglobin; ALB, albumin; OR, odds ratio; CI, confidence interval.
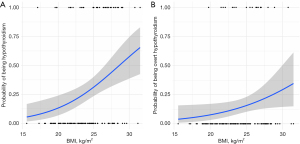
Table 4
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age (continuous) | 1.02 (0.97–1.08) | 0.432 | – | – | |
| Gender (female/male) | 1.24 (0.37–5.65) | 0.749 | – | – | |
| ECOG-PS (0–1/2) | 2.45 (0.71–7.50) | 0.128 | – | – | |
| Smoking (never/current or former) | 2.52 (0.78–11.27) | 0.160 | – | – | |
| Histology (non-squamous/squamous) | 0.96 (0.34–2.54) | 0.938 | – | – | |
| Diabetes (without/with) | 0.29 (0.02–1.54) | 0.241 | – | – | |
| Combined chemotherapy (without/with) | 1.82 (0.70–4.84) | 0.219 | – | – | |
| ICI drug target (PD-L1/PD-1) | 0.36 (0.09–1.79) | 0.167 | – | – | |
| Line of ICI therapy (continuous) | 0.65 (0.32–1.29) | 0.233 | – | – | |
| Best tumor response (PR + SD/PD) | 1.11 (0.24–3.81) | 0.874 | – | – | |
| BMI (continuous) | 1.17 (1.01–1.38) | 0.039* | 1.23 (1.03–1.52) | 0.029* | |
| WBC (continuous) | 1.01 (0.83–1.19) | 0.894 | – | – | |
| NEU (continuous) | 0.95 (0.75–1.03) | 0.503 | – | – | |
| LYM (continuous) | 0.97 (0.65–1.11) | 0.775 | – | – | |
| PLT (continuous) | 1.00 (0.99–1.00) | 0.821 | – | – | |
| MONO (continuous) | 0.81 (0.12–4.36) | 0.818 | – | – | |
| AEC (continuous) | 0.63 (0.04–2.86) | 0.640 | – | – | |
| Hb (continuous) | 1.00 (0.98–1.03) | 0.888 | – | – | |
| ALB (continuous) | 0.96 (0.87–1.06) | 0.393 | – | – | |
*, significant P values <0.05. ECOG-PS, the Eastern Cooperative Oncology Group performance status; PD-L1, programmed cell death protein ligand-1; PD-1, programmed cell death protein-1; ICI, immune checkpoint inhibitor; PR, partial response; SD, stable disease; PD, progressive disease; BMI, body mass index; WBC, white blood cell; NEU, neutrophil; LYM, lymphocyte; PLT, platelet; MONO, monocytes; AEC, absolute eosinophil count; Hb, hemoglobin; ALB, albumin; OR, odds ratio; CI, confidence interval.
Discussion
This study assessed the incidence of hypothyroidism in patients with advanced NSCLC who received PD-1 or PD-L1 inhibitors combined with antiangiogenic therapy, examined the hypothyroidism-related risk factors, and analyzed the effect of baseline BMI on treatment-related hypothyroidism. Overall, 39 cases (28.5%) developed hypothyroidism and 20 cases (14.6%) developed overt hypothyroidism. The observed incidence rate was consistent with those reported in previous studies (18,19,27-29). Our data confirmed no significant increase in the incidence of any grade and overt hypothyroidism in patients who received combination treatment with ICIs and antiangiogenetic agents. Patients with a higher BMI had an increased risk of developing any grade or overt hypothyroidism, which was consistent with the findings of an ICI monotherapy cohort study (19). Our findings suggest that the risk of hypothyroidism with combination therapy might be manageable, mitigating the concern regarding the synergy between the two endocrine toxicity mechanisms.
It is known that hypothyroidism is a common AE during antiangiogenic therapy. Blockade of the VEGF pathway may potentially cause thyroid dysfunction possibly via the following mechanism: Systemic administration of anti-VEGF medications decreases thyroid vascular density and fenestrations, which results in hypothyroidism since VEGF is essential for vascular homeostasis as well as the preservation of vascular integrity and thyroid gland architecture (30). Although hypothyroidism is modest among all irAEs, it is associated with significant morbidity (31,32). The underlying mechanism of ICI-related thyroid dysfunction remains unknown; however, based on the fact that the thyroid gland is more susceptible to autoimmune attacks than any other organ, coupled with the important role of endocrine-specific autoantibodies in the pathogenesis of endocrine-related AEs, it is thought to be caused by impaired the immune tolerance of autoantigens and thyroid cell destruction, similar to autoimmune thyroid disorders (33,34). The use of ICIs combined with antiangiogenic drugs may increase the complexity of toxicity management because some of the AEs of ICIs are similar to those of antiangiogenic therapy. Accurate identification of the cause of each type of hypothyroidism is important but may not always be possible. The identification of risk factors is also crucial to further understanding the mechanism affecting thyroid function and minimizing the treatment-related AEs.
Numerous studies have confirmed the association between obesity and autoimmune thyroid disease, including immunotherapy-related thyroid dysfunction (19,35-38). BMI is an alternative indicator of body fat; one study showed that BMI plays a role in the complex interaction between inflammation and immune dysfunction in patients treated with PD-1 or PD-L1 inhibitors (19). Mechanically, previous studies have shown that the increased prevalence of thyroid dysfunction and thyroid autoantibodies in obese people may be related to increased leptin levels (37) and chronic inflammation caused by obesity (39).
Age has also been thought to be associated with hypothyroidism, possibly because TSH levels increase with age. The underlying mechanism of this relationship may be that the increase of TSH levels in the elderly is due to its decreased biological activity or the reduced thyroid responsiveness to TSH. This is evidenced by the lack of a concurrent decline in T4 levels, suggesting that this is a physiological adaptation process. Therefore, the occurrence of subclinical hypothyroidism in the elderly may have no clinical significance (40). Our results showed that age was only associated with subclinical hypothyroidism but had no predictive significance for overt hypothyroidism, which confirms the above perspective, indicating that steroids or interruption of the combination therapy may not be needed in elderly patients with only a slight increase in TSH.
Several limitations of our study should be noted and considered. Firstly, given that this study was a single-center retrospective study with a relatively small sample size, further research is needed to verify whether the results can be extrapolated to other populations. Secondly, we were unable to obtain the baseline thyroid peroxidase antibodies status of patients, which may limit our investigation into the predictive influence of baseline antibody status on the incidence of hypothyroidism. Thirdly, we did not report on the overall survival of patients in this cohort, and thus, were unable to analyze the relationship between BMI and survival or hypothyroidism and survival. Interestingly, multiple research groups have found an improvement in progression-free and overall survival in obese patients treated with PD-(L)1 checkpoint inhibitors (20,41). Large real-world observational study has also confirmed that baseline obesity is significantly associated with improved objective response rate, progression-free survival, and overall survival in metastatic non-small cell lung cancer patients with a PD-L1 expression of ≥50%, receiving first-line single-agent pembrolizumab, and that there is a significant improvement in ORR, PFS, and OS in patients with slight weight gain during immunotherapy (42). Finally, since the interval between TFTs was not uniform in our study, we were unable to accurately evaluate the time of the initial incidence of hypofunction. Future studies should use multi-center, prospective designs with standardized follow-up intervals to obtain reliable and comprehensive data on hypothyroidism risk in patients receiving immune checkpoint blockade and antiangiogenic therapy, and it would be valuable to investigate the predictive value and mechanisms of obesity on the efficacy and survival of immune checkpoint inhibitors and anti-angiogenic therapy, and assess clinical implications to improve lung cancer treatment outcomes.
Conclusions
Our findings suggest that the risk of hypothyroidism in patients receiving combination treatment with ICIs and antiangiogenic agents is manageable, and a higher BMI is associated with a significantly increased risk of treatment-related hypothyroidism. Clinicians should be aware of these risks and perform regular thyroid function monitoring in high-risk groups during the administration of combination therapy with immune checkpoint blockade and antiangiogenic agents.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of China (No. 82172620, to Richeng Jiang) and the Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-010A).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-306/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-306/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-306/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-306/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional Ethics Committee of Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Tianjin, China (approval number: bc2022098). Due to the anonymity of patient records, the individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pang LL, Gan JD, Huang YH, et al. Role of antiangiogenic agents in first-line treatment for advanced NSCLC in the era of immunotherapy. BMC Cancer 2023;23:72. [Crossref] [PubMed]
- Chen S, Wei H, Zhao W, et al. PD-1/PD-L1 inhibitors plus anti-angiogenic agents with or without chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy as second or later-line treatment for patients with advanced non-small cell lung cancer: A real-world retrospective cohort study. Front Immunol 2022;13:1059995. [Crossref] [PubMed]
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. [Crossref] [PubMed]
- Socinski MA, Nishio M, Jotte RM, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 2021;16:1909-24. [Crossref] [PubMed]
- Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019;20:1109-23. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Phillips AL, Reeves DJ. Outcomes and Management of Immune Checkpoint Inhibitor-Induced Hypothyroidism: A Retrospective Analysis. Ann Pharmacother 2022;56:1100-5. [Crossref] [PubMed]
- Stelmachowska-Banaś M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocr Connect 2020;9:R207-28. [Crossref] [PubMed]
- Torino F, Barnabei A, Paragliola R, et al. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid 2013;23:1345-66. [Crossref] [PubMed]
- Abdel-Rahman O, Fouad M. Risk of thyroid dysfunction in patients with solid tumors treated with VEGF receptor tyrosine kinase inhibitors: a critical literature review and meta analysis. Expert Rev Anticancer Ther 2014;14:1063-73. [Crossref] [PubMed]
- Dote S, Yamaguchi D, Hira D, et al. Thyroid Dysfunction Related to the Antiangiogenic VEGFR2-Binding Monoclonal Antibody Ramucirumab: A Series of 14 Cases and a Descriptive Study. Biol Pharm Bull 2020;43:752-6. [Crossref] [PubMed]
- Gao L, Yang X, Yi C, et al. Adverse Events of Concurrent Immune Checkpoint Inhibitors and Antiangiogenic Agents: A Systematic Review. Front Pharmacol 2019;10:1173. [Crossref] [PubMed]
- Song Y, Fu Y, Xie Q, et al. Anti-angiogenic Agents in Combination With Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front Immunol 2020;11:1956. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol 2018;4:e180013. [Crossref] [PubMed]
- Kobayashi T, Iwama S, Yasuda Y, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc 2018;2:241-51. [Crossref] [PubMed]
- Pollack RM, Kagan M, Lotem M, et al. Baseline TSH level is associated with risk of anti-PD-1-induced thyroid dysfunction. Endocr Pract 2019;25:824-9. [Crossref] [PubMed]
- Pollack R, Ashash A, Cahn A, et al. Immune Checkpoint Inhibitor-induced Thyroid Dysfunction Is Associated with Higher Body Mass Index. J Clin Endocrinol Metab 2020;105:dgaa458. [Crossref] [PubMed]
- Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019;25:141-51. [Crossref] [PubMed]
- Khan SS, Ning H, Wilkins JT, et al. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol 2018;3:280-7. [Crossref] [PubMed]
- Kaaks R, Kühn T. Epidemiology: obesity and cancer--the evidence is fattening up. Nat Rev Endocrinol 2014;10:644-5. [Crossref] [PubMed]
- Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002;3:565-74. [Crossref] [PubMed]
- Abdullah A, Peeters A, de Courten M, et al. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 2010;89:309-19. [Crossref] [PubMed]
- Narayan KM, Boyle JP, Thompson TJ, et al. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007;30:1562-6. [Crossref] [PubMed]
- World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment. 2000.
- Brody HM, Macherla S, Bulumulle A, et al. The real-world incidence of immunotherapy-related thyroid dysfunction: A retrospective analysis of a single center’s experience over five years. J Clin Oncol 2020;38:abstr 98.
- Morganstein DL, Lai Z, Spain L, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf) 2017;86:614-20. [Crossref] [PubMed]
- Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid 2020;30:177-84. [Crossref] [PubMed]
- Cao Y. VEGF-targeted cancer therapeutics-paradoxical effects in endocrine organs. Nat Rev Endocrinol 2014;10:530-9. [Crossref] [PubMed]
- Weinstein A, Gordon RA, Kasler MK, et al. Understanding and Managing Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors in Patients With Advanced Melanoma. J Adv Pract Oncol 2017;8:58-72. [PubMed]
- So AC, Board RE. Real-world experience with pembrolizumab toxicities in advanced melanoma patients: a single-center experience in the UK. Melanoma Manag 2018;5:MMT05. [Crossref] [PubMed]
- Luty J, Ruckemann-Dziurdzińska K, Witkowski JM, et al. Immunological aspects of autoimmune thyroid disease - Complex interplay between cells and cytokines. Cytokine 2019;116:128-33. [Crossref] [PubMed]
- Labadzhyan A, Wentzel K, Hamid O, et al. Endocrine Autoantibodies Determine Immune Checkpoint Inhibitor-induced Endocrinopathy: A Prospective Study. J Clin Endocrinol Metab 2022;107:1976-82. [Crossref] [PubMed]
- Wang S, Baidoo SE, Liu Y, et al. T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto’s thyroiditis. Clin Exp Immunol 2013;171:63-8. [Crossref] [PubMed]
- Ong KK, Kuh D, Pierce M, et al. Childhood weight gain and thyroid autoimmunity at age 60-64 years: the 1946 British birth cohort study. J Clin Endocrinol Metab 2013;98:1435-42. [Crossref] [PubMed]
- Marzullo P, Minocci A, Tagliaferri MA, et al. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab 2010;95:3965-72. [Crossref] [PubMed]
- Kang C, Liu J, Zheng Y, et al. Association of high BMI with subclinical hypothyroidism in young, first-episode and drug-naïve patients with major depressive disorder: a large-scale cross-sectional study. Eur Arch Psychiatry Clin Neurosci 2023;273:183-90. [Crossref] [PubMed]
- Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol 2014;220:T47-59. [Crossref] [PubMed]
- Zhai X, Zhang L, Chen L, et al. An Age-Specific Serum Thyrotropin Reference Range for the Diagnosis of Thyroid Diseases in Older Adults: A Cross-Sectional Survey in China. Thyroid 2018;28:1571-9. [Crossref] [PubMed]
- Naik A, Monjazeb AM, Decock J. The obesity paradox in cancer, tumor immunology, and immunotherapy: potential therapeutic implications in triple negative breast cancer. Frontiers in immunology. 2019;10:1940. [Crossref] [PubMed]
- Cortellini A, Ricciuti B, Tiseo M, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression ≥ 50%: a multicenter study with external validation. J Immunother Cancer 2020;8:e001403. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


