
Efficacy and safety of robotic-assisted versus median sternotomy for cardiac surgery: results from a university affiliated hospital
Highlight box
Key findings
• The Da Vinci robotic surgery system is safe and effective for cardiac surgery.
What is known and what is new?
• As an innovative minimally invasive surgery, robotic surgery has gradually been applied in the field of cardiac surgery.
• This study collects the clinical data of all patients undergoing robotic heart surgery in our center and compares them with patients undergoing traditional surgery to evaluate their effectiveness and safety.
What is the implication, and what should change now?
• The results of this study show that use of the Da Vinci robotic surgery system to assist cardiac surgery is safe and effective, which can not only shorten the ICU time and postoperative hospital stay, but also improve patient satisfaction and postoperative quality of life. There is a certain learning curve. The application of robotics in cardiac surgery is worth promoting.
Introduction
With the development of medical technology, all kinds of minimally invasive cardiac surgery techniques emerge as the times require, and the traditional thoracotomy has been gradually replaced because of its large trauma and slow recovery. Robotic surgery can effectively reduce trauma and improve the quality of life of patients after operation, which provides a new choice for the treatment of patients with heart disease. Robotic surgery systems represent a revolutionary development for the application of minimally invasive techniques in cardiac surgery. By virtue of the flexibility and 3-dimensional (3D) visualization of wrist-type devices, robotic techniques allow the advantages of the human wrist and eye to be applied in the field of minimally invasive surgery (1-3). In 1998, Carpentier reported the first successful mitral valve surgery performed independently using the prototype of the Da Vinci robotic surgery system (4,5). Since then, the first generation of the Da Vinci robotic surgery system has been developed and approved by the U.S. Food and Drug Administration (FDA) for clinical application. Our hospital closely followed the cutting-edge of minimally invasive robotics and completed the first case of robotic atrial septal defect (ASD) repair surgery in July 2017. By the end of May 2022, 255 cases of robotic cardiac surgery had been completed in our hospital. In this study, the clinical data of all patients who underwent robotic cardiac surgery in the First Affiliated Hospital of Anhui Medical University were collected. After comparison with patients who underwent traditional surgery, the efficacy and safety of robotic cardiac surgery were evaluated. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-197/rc).
Methods
General information
This study is a clinical retrospective cohort study. A total of 255 patients received cardiac surgery assisted by the Da Vinci robotic surgery system from July 2017 to May 2022 in the First Affiliated Hospital of Anhui Medical University, and they were defined as the robotic-assisted cardiac surgery (RACS) group. And, 736 patients with the same disease types who underwent median sternotomy and had complete data in the same period were selected as the traditional open-heart surgery (TOHS) group. The inclusion criterion of RACS group include: (I) age >18 years; (II) selective surgery; (III) non-aortic surgery; (IV) no serious pleural adhesion; (V) good lung function which can tolerate one-lung ventilation; (VI) suitable femoral artery diameter. In relation to TOHS group, the selection of patients was by reviewing the hospital electronic medical record information system. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of First Affiliated Hospital of Anhui Medical University (No. PJ2022-06-20) and individual consent for this retrospective analysis was waived.
All RACS operations were performed by the same surgeon who used the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA). Detailed surgical procedures were provided in the Appendix 1.
Follow-up
All patients underwent the first outpatient follow-up 1 month after discharge, including blood routine, biochemical function, coagulation function, and echocardiography. Also, the time taken for patients to return to normal daily activities was assessed. Subsequent follow-up was completed by clinic visit or telephone contact according to the specific situation of each patient.
Outcome measures
We compared all intra- and postoperative data between both groups. Among them, we focused on the following variables, comprising surgery time, reoperation rate for postoperative bleeding, length of intensive care unit (ICU) stay, postoperative hospitalization day, the numbers of died and withdrawing treatments, and the time of patients back to normal daily activities after discharge.
Statistical analysis
The normality of continuous data was tested, if the data conforms to normal distribution, it was expressed as mean ± standard deviation and Student’s t-test was used to compare the difference between both groups, otherwise, it was expressed as median value (M) and interquartile range (IQR) and compared using Mann-Whitney rank sum test. Categorical variables were presented as n (%) and rates between groups and were compared using the chi-square test or Fisher’s exact test. Time-event data was defined as survival data and the Kaplan-Meier (K-M) method was utilized for comparison. We used multivariate Cox regression analysis to assess the relationship between RACS with the time of patients returning to normal daily activities after surgery. Variables were selected based on clinical reality and previous published articles. Finally, 7 variables were entered into the regression analysis model, including gender, age, preoperative cardiac function [New York Heart Association Functional Classification (NYHA classification)], left ventricular ejection fraction (LVEF), surgery time, presence of comorbidities, and surgery methods. Data analysis was performed using SPSS 16.0 version statistical software (IBM Corp., Armonk, NY, USA) package, and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) was employed for plotting. In this study, 2-sided tests were applied, and P<0.05 was considered statistically significant.
Results
The learning curve was constructed according to surgery time and the time for preparing robotic surgical equipment. At present, the mean time of the aforementioned two indices was 3 hours and 30 minutes, respectively (Figure 1). The baseline data of RACS and TOHS group are shown in Table 1, and the comparison of each disease type between the 2 groups is displayed in Table 2. In the RACS group, 2 cases scheduled for mitral valvuloplasty (MVP) were changed to mitral valve replacement (MVR) due to unsatisfactory intraoperative effect; 1 patient undergoing ASD repair developed abdominal hemorrhage on account of a rupture in the dorsal side of the infrarenal abdominal aorta, however, the rescue was invalid and the patient eventually died; after robotic MVP, liver bleeding occurred in 1 patient because a mechanical arm punctured his diaphragm, through laparotomy, the bleeding was stopped, and the patient was recovered and discharged; 1 case experiencing bleeding induced by Trocar site hernia was cured and discharged after hemostasis; the healing of the 4th intercostal incision for 2 patients after coronary artery bypass (CAB) grafting was delayed; 1 hypertrophic obstructive cardiomyopathy (HOCM) patient experienced severe pulmonary infection secondary to diffuse alveolar hemorrhage upon surgery, for which he left hospital without having achieved a cure.
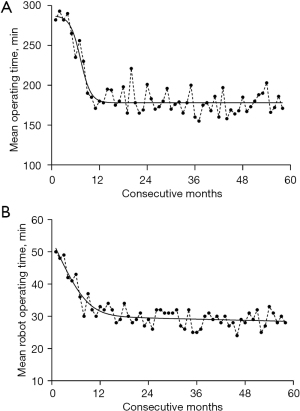
Table 1
| Index | RACS group (N=255) | TOHS group (N=736) | t/χ2 value | P value |
|---|---|---|---|---|
| Age (year) | 52.2±6.1 | 53.1±7.0 | −1.827 | 0.068 |
| Gender (female/male) | 134/121 | 351/385 | 1.789 | 0.181 |
| BMI (kg/m2) | 20.8±2.4 | 20.5±2.2 | 1.839 | 0.066 |
| LVEF | 62.3±2.1 | 62.5±2.0 | −1.358 | 0.175 |
| NYHA classification | 5.831 | 0.120 | ||
| I stage | 92 | 240 | ||
| II stage | 87 | 313 | ||
| III stage | 73 | 176 | ||
| IV stage | 3 | 7 | ||
| Diabetes | 49 | 119 | 1.249 | 0.246 |
| Hypertension | 67 | 174 | 0.713 | 0.398 |
| COPD | 26 | 59 | 1.148 | 0.284 |
Data are presented as number or mean ± SD. RACS, robotic-assisted cardiac surgery; TOHS, traditional open-heart surgery; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease.
Table 2
| Parameters | MIDCAB | ASD repair | ASD repair + PAPVC correction | VSD repair# | Atrial tumor resection | Pericardiectomy | MVP | MVR | HOCM correction |
|---|---|---|---|---|---|---|---|---|---|
| RACS group | n=34 | n=101 | n=3 | n=1 | n=30 | n=2 | n=41 | n=27 | n=16 |
| Age (year) | 65.4±7.3 | 31.1±6.5 | 33.3±4.1 | 35 | 46.5±5.3 | 41 | 56.3±7.3 | 59.4±6.7 | 49.8±11.4 |
| Gender (male/female) | 19/15 | 43/58 | 1/2* | 1/0 | 18/12 | 2/0* | 23/18 | 17/10 | 10/6 |
| BMI (kg/m2) | 21.8±2.3 | 20.2±1.9 | 20.1±2.7 | 21.2 | 21.4±2.2 | 20.6 | 21.4±2.1 | 21.5±2.1 | 21.7±2.1 |
| LVEF | 57.23±2.78 | 63.24±3.34 | 62.84±2.99 | 62 | 61.47±1.89 | 62.5 | 59.48±3.29 | 60.39±2.43 | 62.54±2.39 |
| NYHA classification | |||||||||
| I stage | 4 | 71 | 2 | 1 | 5 | 2 | 2 | 5 | 0 |
| II stage | 18 | 20 | 1 | 0 | 15 | 0 | 25 | 15 | 3 |
| III stage | 12 | 10 | 0 | 0 | 10 | 0 | 14 | 7 | 10 |
| IV stage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Complications | |||||||||
| Diabetes | 24 | 5 | 0 | 0 | 3 | 0 | 5 | 7 | 2 |
| Hypertension | 28 | 3 | 0 | 1 | 5 | 1 | 15 | 11 | 3 |
| COPD | 16 | 0 | 0 | 0 | 1 | 0 | 3 | 5 | 1 |
| TOHS group | n=40 | n=162 | n=20 | n=37 | n=43 | n=11 | n=139 | n=262 | n=22 |
| Age (year) | 64.5±7.1 | 33.1±5.3 | 33.6±4.7 | 34.2±5.0 | 43.5±4.3 | 41.8±4.7 | 56.4±7.36 | 58.9±6.3 | 49.68±10.4 |
| Gender (male/female) | 22/18 | 73/89 | 11/9* | 17/20 | 20/23 | 7/4* | 66/73 | 128/134 | 15/7 |
| BMI (kg/m2) | 21.3±2.1 | 20.4±2.1 | 21.1±2.6 | 20.8±2.4 | 21.2±2.9 | 20.5±2.7 | 21.5±2.4 | 21.4±2.0 | 21.4±2.2 |
| LVEF | 56.23±2.37 | 63.55±3.37 | 63.84±2.72 | 62.53±2.1 | 61.40±1.75 | 62.12±1.68 | 59.68±3.38 | 60.45±2.38 | 62.48±2.58 |
| NYHA classification | |||||||||
| I stage | 6 | 110 | 11 | 28 | 8 | 11 | 7 | 51 | 0 |
| II stage | 21 | 39 | 8 | 9 | 20 | 0 | 80 | 147 | 5 |
| III stage | 13 | 13 | 1 | 0 | 15 | 0 | 52 | 60 | 14 |
| IV stage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 3 |
| Complications | |||||||||
| Diabetes | 20 | 10 | 3 | 0 | 3 | 0 | 17 | 63 | 3 |
| Hypertension | 23 | 5 | 2 | 0 | 6 | 3 | 52 | 86 | 3 |
| COPD | 17 | 0 | 0 | 0 | 1 | 0 | 12 | 27 | 2 |
Data are presented as number or mean ± SD. *, P<0.05; #, no statistical comparison of sample data. MIDCAB, minimally invasive direct coronary artery bypass; ASD, atrial septal defect; PAPVC, partial anomalous pulmonary venous connection; VSD, ventricular septal defect; MVP, mitral valvuloplasty; MVR, mitral valve replacement; HOCM, hypertrophic obstructive cardiomyopathy; RACS, robotic-assisted cardiac surgery; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; TOHS, traditional open heart surgery.
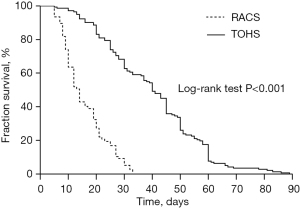
The comparison of intraoperative and postoperative indexes between the 2 groups is shown in Table 3. Specifically, the RACS group patients presented longer total surgery time, cardiopulmonary bypass (CPB) time, and aortic cross-clamp time while less postoperative ICU length of stay, total hospital stay, postoperative 24-hour drainage volume, and cases of blood transfusions than compared with the TOHS group. However, there was no statistical difference in reoperation rate for postoperative bleeding, rate of patients who withdrew treatments, and mortality between the 2 groups. The comparison of indexes between different disease types is displayed in Table 4. Due to few patients with ventricular septal defect (VSD) repair (n=1), pericardiectomy (n=2), and ASD repair + partial anomalous pulmonary venous connection (PAPVC) correction (n=3) in the TOHS group, the above 3 disease types were not significantly different. Hence, only relevant indexes were described for patients with VSD, pericardial cyst, and ASD+PAPVC in the TOHS group. Relative to the TOHS group, the RACS group exhibited slightly longer average time of the surgery and CPB for patients undergoing VSD repair and ASD repair + PAPVC correction and almost similar average surgery time for those receiving pericardiectomy. Besides, the RACS group patients with the above 3 disease types showed slightly shorter length of ICU stay, postoperative 24-hour drainage volume, and postoperative hospital stay in comparison with the TOHS group patients. Furthermore, matched against the TOHS group, patients who underwent atrial tumor resection, HOCM correction, MVP, and MVR in the RACS group displayed longer surgery time, CPB time, and aortic cross-clamp time yet shorter postoperative ICU length of stay, 24-hour drainage volume, and cases of blood transfusions, with significant differences between the 2 groups. Moreover, the surgery time of patients receiving minimally invasive direct coronary artery bypass (MIDCAB) in the RACS group was longer than those in the TOHS group, but the difference was not statistically significant, and none of them required blood transfusion. Additionally, the mean surgery and CPB time of patients who received ASD repair alone in the RACS group were longer than those in the TOHS group whereas the postoperative ICU length of stay and 24-hour drainage volume were lower, and the difference between the 2 groups was statistically significant. Overall, postoperative complications between the RACS group and the TOHS group were not significantly different. The K-M curve analysis revealed that patients in the RACS group had a significantly shorter time to return to normal daily activities after surgery than those in the TOHS group (log-rank P<0.001, Figure 2). A multivariate Cox regression analysis also reinforced this finding after entering covariates into the model to adjust.
Table 3
| Indexes | RACS group (N=255) | TOHS group (N=736) | t/χ2 value | P value |
|---|---|---|---|---|
| Surgery time (min) | 215.6±17.5 | 190.8±18.4 | 18.780 | <0.001 |
| CPB time# (min) | 142.3±15.6 | 130.2±15.1 | 10.933 | <0.001 |
| Aortic cross-clamp time# (min) | 115.5±16.2 | 101.7±14.3 | 12.822 | <0.001 |
| ICU length of stay (hours) | 30.8±5.6 | 35.7±8.9 | −8.243 | <0.001 |
| Postoperative 24-hour drainage volume (mL) | 253.6±35.6 | 319.8±41.9 | −22.564 | <0.001 |
| Postoperative hospital stay (days) | 8.4±1.8 | 10.7±5.5 | −6.555 | <0.001 |
| Cases of blood transfusion | 95 | 340 | 6.147 | 0.013 |
| Reoperation for postoperative bleeding | 2 | 6 | 0.000 | 1.000 |
| Patients left hospital without cure | 1 | 7 | 0.857 | 0.354 |
| Death | 1 | 0 | * | 0.257 |
Data are presented as number or mean ± SD. #, patients without cardiopulmonary bypass were not included in the comparison; *, data calculated by Fisher exact test and without specific statistics. RACS, robotic-assisted cardiac surgery; TOHS, traditional open-heart surgery; CPB, cardiopulmonary bypass; ICU, intensive care unit.
Table 4
| Indexes | MIDCAB | ASD repair | ASD repair + PAPVC correction# | VSD repair# | Atrial tumor resection | Pericardiectomy# | MVP | MVR | HOCM correction |
|---|---|---|---|---|---|---|---|---|---|
| RACS group | n=34 | n=101 | n=3 | n=1 | n=30 | n=2 | n=41 | n=27 | n=16 |
| Surgery time (min) | 180.8±16.6 | 190.4±12.68 | 205.0±11.8 | 210 | 195.0±18.8* | 95 | 249.1±25.8* | 217.7±14.6* | 314.3±16.7* |
| CPB time (min) | 0 | 69.3±7.8* | 79.7±5.7 | 90 | 107.4±14.3* | 0 | 131.2±21.3* | 160.2±18.9* | 241.0±9.5* |
| Aortic cross-clamp time (min) | 0 | 0 | 0 | 56 | 64.3±6.0* | 0 | 112.0±15.2 | 114.6±16.5* | 166.3±15.6 |
| ICU length of stay (hours) | 18.9±4.9 | 18.2±4.6* | 20.7±3.3 | 22 | 22.6±3.3* | 0 | 29.9±4.1* | 34.3±3.8* | 66.7±11.6* |
| Postoperative 24-hour drainage volume (mL) | 118.2±17.5 | 195.6±24.3* | 173.0±30.7 | 210 | 242.8±26.6* | 164 | 289.3±44.0* | 319.0±30.3* | 424.7±51.8* |
| Postoperative hospital stay (days) | 7.1±2.0 | 8.0±1.9* | 7.3±1.2 | 8 | 7.3±1.2* | 5.5 | 8.3±1.9* | 8.3±1.8* | 10.5±1.7* |
| Cases of blood transfusion | 0 | 3* | 0 | 0 | 18* | 0 | 41* | 27* | 16 |
| Patients left hospital without cure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Reoperation for postoperative bleeding | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Death | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TOHS group | n=40 | n=162 | n=20 | n=37 | n=43 | n=11 | n=139 | n=262 | n=22 |
| Surgery time (min) | 179.1±16.6 | 181.4±12.3* | 200.0±9.8 | 205.8±5.9 | 181.0±16.8* | 80.5±6.7 | 226.1±21.8* | 205.7±13.6* | 300.3±11.7* |
| CPB time (min) | 0 | 60.3±7.8* | 70.7±8.7 | 85.8±6.9 | 92.4±11.3* | 0 | 120.2±18.3* | 133.2±17.9* | 215.5±7.9* |
| Aortic cross-clamp time (min) | 0 | 0 | 0 | 40.6±8.5 | 57.2±6.0* | 0 | 91.0±14.2* | 93.6±16.1* | 143.3±11.6* |
| ICU length of stay (hours) | 22.8±4.6* | 23.5±5.6* | 22.7±3.5 | 25.3±3.8 | 25.6±3.8* | 0 | 35.5±6.1* | 36.7±4.2* | 72.6±17.6* |
| Postoperative 24-hour drainage volume (mL) | 140.2±20.5* | 220.5±20.3* | 200.0±35.7 | 220.5±6.9 | 260.8±30.6* | 190.8±20.6 | 310.5±36.8* | 330.8±26.3* | 441.7±60.2* |
| Postoperative hospital stay (days) | 8.1±2.2 | 10.0±1.5* | 8.3±2.2 | 9.5±1.8 | 10.3±1.8* | 6.5±2.1 | 11.5±2.2* | 12.1±2.1* | 12.7±2.8* |
| Cases of blood transfusion | 0 | 10* | 0 | 0 | 25* | 0 | 93* | 192* | 20 |
| Patients left hospital without cure | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 | 0 |
| Reoperation for postoperative bleeding | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Data are presented as number or mean ± SD. *, P<0.05; #, no statistical comparison of sample data. MIDCAB, minimally invasive direct coronary artery bypass; ASD, atrial septal defect; PAPVC, partial anomalous pulmonary venous connection; VSD, ventricular septal defect; MVP, mitral valvuloplasty; MVR, mitral valve replacement; HOCM, hypertrophic obstructive cardiomyopathy; RACS, robotic-assisted cardiac surgery; CPB, cardiopulmonary bypass; ICU, intensive care unit; TOHS, traditional open heart surgery.
Postoperative follow-up lasted for 1–53 months. To be specific, 1 patient died of intraoperative abdominal aortic rupture and bleeding, 1 patient died of HOCM after leaving hospital without cure, and other patients were cured and discharged. Besides, 24 of the 34 patients who underwent CAB grafting completed coronary computed tomography angiography (CTA) after surgery, all of which revealed unobstructed left internal mammary artery (LiMA) graft vessels. Moreover, follow-up color Doppler ultrasound indicated that there was no moderate or above mitral regurgitation in patients with mitral valve repair, and no residual shunt or myxoma recurrence in patients with ASD repair and VSD repair. In addition, patients receiving robotic surgery remained in follow-up.
Discussion
Based on the development of minimally invasive techniques, endoscopic minimally invasive surgery is becoming preferred for various surgical treatments and has achieved significant results. Currently, endoscopic minimally invasive surgery is emerging as the preferred clinical program for various cardiac surgical diseases (6). Robotic surgery, as an innovative minimally invasive surgery, has been gradually applied in the field of cardiac surgery (7). Assessment for the vascular conditions of patients is necessary. In this study, 1 patient died of abdominal aortic rupture. A small ulcer surrounded by a few plaques was observed in the abdominal aorta of the patient, which may be the cause of abdominal aortic rupture during intraoperative CPB.
Khalafallah et al. (8) pointed out that the Da Vinci robot had a shorter learning curve and performed better in difficult surgeries than traditional thoracoscopy. Especially, when complex procedures and multidimensional multiangle suture are performed in the thoracic narrow space, traditional open and thoracoscopic surgeries are prone to vascular injury or myocardial injury. The Da Vinci robotic surgical system has the advantages of reducing surgical difficulty, improving surgical accuracy, alleviating operator fatigue, compensating for the limitations of endoscopic techniques, expanding surgical indications, and making difficult cardiac surgery possible. Güllü et al. (9) stated that the Da Vinci robot had advantages in terms of intraoperative blood loss, length of hospital stay, and complication rate in cardiac surgery compared with thoracoscopy. Van den Eynde et al. (10) claimed that the Da Vinci robotic surgery could significantly shorten the hospital stay of patients and promote their early functional rehabilitation. Dang et al. (11) reported the efficacy and safety of totally endoscopic cardiac surgery for ASD repair on a beating heart without robotic assistance in 25 patients, with an average surgery time of 267.2±44.6 minutes, an average CPB time of 156.1±33.6 minutes, and 1 week after surgery, patients could return to the normal activities. In this article, the time of surgery and CPB for patients with ASD were shorter than that reported, indicating certain clinical advantages of robotic surgery over thoracoscopic surgery in promoting early functional rehabilitation of patients. Yanagawa et al. (12) analyzed the results of 5,199 patients who underwent robotic cardiac surgery and found that these patients tended to match those who underwent more traditional, non-robotic cardiac surgery. Yanagawa et al. (12) discovered that patients who received robotic surgery had significantly lower mortality, shorter hospital stays, and fewer overall perioperative complications compared with those who underwent non-robotic surgery.
Robotic cardiac surgeries performed worldwide mainly include robotic CAB surgery and robotic mitral valve surgery. Robotic MIDCAB can be used in patients with independent left anterior descending (LAD) disease or multivessel coronary artery stenosis, and combined with percutaneous techniques, it can be also applied in all non-LAD diseased vessels; the latter approach constitutes a hybrid coronary revascularization (HCR) strategy (13). The 2012 American Heart Association (AHA)/Society of Thoracic Surgeons (STS) guideline for the diagnosis and management of patients provides a level IIb recommendation for HCR, and indicates that HCR may be a reasonable choice for MIDCAB or multivessel percutaneous coronary intervention (PCI), thereby improving the risk-benefit ratio of the procedure (14). The 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines for myocardial revascularization mention that HCR can serve as an option when multivessel PCI was considered inappropriate, or when CAB grafting was believed high risk.
There is little doubt about the benefits of robotic-assisted mitral valve surgery. Numerous reports have demonstrated that robot-assisted surgery reduces perioperative complications, decreases transfusion requirements, shortens mechanical ventilation time, reduces ICU and overall hospital stays, alleviates postoperative pain, enables patients to return to normal activities more quickly, and increases patients’ overall satisfaction (15,16).
Cao et al. (17) performed a meta-analysis of 6 studies comparing robotic and conventional mitral valve surgery and demonstrated that robotic mitral valve surgery was safe and had a low perioperative complication rate, particularly if performed by experienced surgeons, despite a long CPB time and myocardial cross-clamp time.
In an analysis of the STS adult cardiac surgery database (USA), Wang et al. (18) reported the results of 503 patients who underwent robotic mitral valve repair, matching a concurrent group of valve repair by median sternotomy. Briefly, robotic therapy was associated with reduced postoperative atrial fibrillation, lower transfusion rates, and shorter hospital stays, and the 3-year mortality of the above 2 groups was similar. Importantly, there was no significant difference in the need for valve reintervention during mid-term follow-up, which has been reconfirmed by another study (19). However, compared with the median sternotomy group, CPB time and myocardial cross-clamp time were longer in the robotic group.
Potential concerns for robotic technology include longer time of CBP and myocardial ischemia, which have been mentioned in almost all studies. It is reported that (19) these concerns seem to improve over time because surgeons become more surgically proficient (20). In addition, longer perfusion time does not seem to be directly related to increased perioperative morbidity (21,22). Concerns about the increased risk of perioperative stroke associated with peripheral extracorporeal circulation cannulation can be greatly mitigated by appropriate preoperative screening for atherosclerotic disease, suitable patient selection, and replacement of cannula position as needed.
A large number of literature reports that almost all types of ASD have been successfully repaired with robot-assisted surgery, including coronary sinus defects with PAPVC drainage; the setting and operation of robotic arms are similar to robotic mitral valve surgery (23). Also, a study has reported successful robot-assisted partial endocardial cushion defect repair and an atrioventricular septal defect malformation associated with trisomy 21 (24).
Left atrial myxoma is the most common intracardiac tumor successfully resected using robotic technology. Essentially, robot-assisted left atrial myxoma resection is a surgical technique that mimics mitral valve surgery. Advantages of the robotic approach include improving surgical exposure, shortening hospital stay, reducing postoperative pain, and quickening functional recovery. Myxomas have also been reported to be successfully resected from other cardiac chambers, including the right atrium and left ventricle (25).
Although robotic mitral valve surgery is common now, the application of robotic surgery in the aortic valve is not popularized due to some limitations. There is a single case report of aortic valve replacement (AVR) with robotic assistance (26) and a study on successful robot-assisted aortic valve papillary fibroelastoma resection (27). However, robotic surgical procedures for aortic valve are still not performed in our hospital.
The main limitation of this study is the comparability of baseline data between both groups. Comparability is extremely important for a rigorous paper, but unfortunately, although propensity score matching method may be a good choice, it is hard for us to do this because limited sample size and different disease types. According to our knowledge, IPTW method is another alternative as it can do post-randomization; however, because of the virtual sample size, its result may cause serious bias. In this study, we directly compared the clinical results between the two groups, and reasons are as follows. First, for eligible patients, the advantages of RACS are self-evident compared with TOHS, furthermore, a large number of published articles have also proved it. Second, there is no significant difference when comparing all baseline variables that we collected, this may be a natural randomization of the real world and indicated that our results are credible.
In summary, robotic surgery is a promising technology. With the development of minimally invasive surgical systems, robots can help surgeons with complex cardiac surgery, not limited to fixed disease types.
Conclusions
This study has preliminarily displayed the safety and effectiveness of Da Vinci robotic surgery system assisted cardiac surgery. On the one hand, robotic surgery shortens the ICU time and postoperative hospital stay; on the other hand, it improves patients’ satisfaction and postoperative quality of life. Moreover, the Da Vinci robotic surgery system is characterized by a certain learning curve. In a word, the application of robots in cardiac surgery is worthy of promotion.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-197/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-197/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-197/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-197/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee of First Affiliated Hospital of Anhui Medical University (No. PJ2022-06-20) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hemli JM, Patel NC. Robotic Cardiac Surgery. Surg Clin North Am 2020;100:219-36. [Crossref] [PubMed]
- Harky A, Chaplin G, Chan JSK, et al. The Future of Open Heart Surgery in the Era of Robotic and Minimal Surgical Interventions. Heart Lung Circ 2020;29:49-61. [Crossref] [PubMed]
- Balkhy HH, Amabile A, Torregrossa G. A Shifting Paradigm in Robotic Heart Surgery: From Single-Procedure Approach to Establishing a Robotic Heart Center of Excellence. Innovations (Phila) 2020;15:187-94. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Aupècle B, et al. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Chitwood WR Jr. Historical evolution of robot-assisted cardiac surgery: a 25-year journey. Ann Cardiothorac Surg 2022;11:564-82. [Crossref] [PubMed]
- Hosoba S, Ito T, Orii M. Three-Dimensional Endoscopic-Assisted Concomitant Mitral and Aortic Valve Surgery. Ann Thorac Surg 2022;114:e63-6. [Crossref] [PubMed]
- Jia YX, Zhang YJ, Li HC. Applications and advances of robotic surgical system in thoracic surgery. Chinese Journal of Robotic Surgery 2022;3:367-75.
- Khalafallah YM, Bernaiche T, Ranson S, et al. Residents' Views on the Impact of Robotic Surgery on General Surgery Education. J Surg Educ 2021;78:1007-12. [Crossref] [PubMed]
- Güllü AÜ, Şenay Ş, Ersin E, et al. Robotic-assisted cardiac surgery without aortic cross-clamping: A safe alternative approach. J Card Surg 2021;36:165-8. [Crossref] [PubMed]
- Van den Eynde J, Melly L, Torregrossa G, et al. Robotic Cardiac Surgery: What the Young Surgeon Should Know. Braz J Cardiovasc Surg 2020;35:VI-VIII. [PubMed]
- Dang QH, Le NT, Nguyen CH, et al. Totally Endoscopic Cardiac Surgery for Atrial Septal Defect Repair on Beating Heart Without Robotic Assistance in 25 Patients. Innovations (Phila) 2017;12:446-52. [Crossref] [PubMed]
- Yanagawa F, Perez M, Bell T, et al. Critical Outcomes in Nonrobotic vs Robotic-Assisted Cardiac Surgery. JAMA Surg 2015;150:771-7. [Crossref] [PubMed]
- Hemli JM, Patel NC, Subramanian VA. Increasing surgical experience with off-pump coronary surgery does not mitigate the morbidity of emergency conversion to cardiopulmonary bypass. Innovations (Phila) 2012;7:259-65. [Crossref] [PubMed]
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354-471. [PubMed]
- Murphy DA, Moss E, Binongo J, et al. The expanding role of endoscopic robotics in mitral valve surgery: 1257 consecutive procedures. J Thorac Cardiovasc Surg 2015;100:1675-81; discussion 1681-2. [PubMed]
- Breves SL, Hong I, McCarthy J, et al. Ascending Aortic Endoballoon Occlusion Feasible Despite Moderately Enlarged Aorta to Facilitate Robotic Mitral Valve Surgery. Innovations (Phila) 2016;11:355-9. [Crossref] [PubMed]
- Cao C, Wolfenden H, Liou K, et al. A meta-analysis of robotic vs. conventional mitral valve surgery. Ann Cardiothorac Surg 2015;4:305-14. [PubMed]
- Wang A, Brennan JM, Zhang S, et al. Robotic Mitral Valve Repair in Older Individuals: An Analysis of The Society of Thoracic Surgeons Database. Ann Thorac Surg 2018;106:1388-93. [Crossref] [PubMed]
- Liu G, Zhang H, Yang M, et al. Robotic mitral valve repair: 7-year surgical experience and mid-term follow-up results. J Cardiovasc Surg (Torino) 2019;60:406-12. [Crossref] [PubMed]
- Yaffee DW, Loulmet DF, Kelly LA, et al. Can the learning curve of totally endoscopic robotic mitral valve repair be short-circuited? Innovations (Phila) 2014;9:43-8. [Crossref] [PubMed]
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [Crossref] [PubMed]
- Hawkins RB, Mehaffey JH, Mullen MG, et al. A propensity matched analysis of robotic, minimally invasive, and conventional mitral valve surgery. Heart 2018;104:1970-5. [Crossref] [PubMed]
- Gao C, Yang M, Wang G, et al. Totally robotic resection of myxoma and atrial septal defect repair. Interact Cardiovasc Thorac Surg 2008;7:947-50. [Crossref] [PubMed]
- Gao C, Yang M, Xiao C, et al. Totally Endoscopic Robotic Correction of Cor Triatriatum Sinister Coexisting With Atrial Septal Defect. Innovations (Phila) 2016;11:451-2. [Crossref] [PubMed]
- Onan B, Kahraman Z, Erturk M, et al. Robotic resection of giant left ventricular myxoma causing outflow tract obstruction. J Card Surg 2017;32:281-4. [Crossref] [PubMed]
- Folliguet TA, Vanhuyse F, Konstantinos Z, et al. Early experience with robotic aortic valve replacement. Eur J Cardiothorac Surg 2005;28:172-3. [Crossref] [PubMed]
- Nisivaco SM, Patel B, Balkhy HH. Robotic totally endoscopic excision of aortic valve papillary fibroelastoma: The least invasive approach. J Card Surg 2019;34:1492-7. [Crossref] [PubMed]
(English Language Editor: J. Jones)


