Pitfalls in oncology: a unique case of thoracic splenosis mimicking malignancy in a patient with resected breast cancer
Introduction
Thoracic splenosis (TS) is a condition of ectopic splenic tissue into the pleural cavity or, very rarely, pulmonary parenchyma after thoraco-abdominal trauma with spleen and diaphragmatic injury. In this clinical setting, its prevalence is nearly 18% of cases (1). The median time between trauma and its radiological detection is 21 years (2). Usually asymptomatic, TS is discovered as an incidental finding at imaging performed for other reasons and has to be differentiated from other benign and malignant conditions (3). Its diagnosis should be reached avoiding invasive procedures. We report a case of thoracic mass associated with pleural nodules mimicking malignancy in a patient with resected breast cancer for whom a diagnosis of TS was made early by using non-invasive methods. Briefly, we review the literature data on TS, comment concisely the possible implications of using invasive procedures and describe the current non-invasive techniques available. Furthermore, we highlight the importance of an accurate medical history collection, the role of the multidisciplinary board and their impact on treatment decision making. Finally, we conclude that clinical information and imaging would be the discriminating factors to avoid unnecessary invasive procedures.
Case presentation
A 51-year-old Caucasian woman was referred to our Institution for the evaluation of adjuvant therapies after a radical left mastectomy for breast carcinoma. Pathology report was consistent with a moderately differentiated (grade 2) lobular carcinoma, pT2 (4 cm in maximum diameter), no blood vessel invasion, pN3 (14 out of 16 lymph nodes sampled), ER and PgR positive (66–100%), p185/Her2neu 1+, proliferative index (Ki67) of 10%.
Patient’s medical history was significant for hypertension, insulin-dependent diabetes mellitus and moderate chronic renal failure.
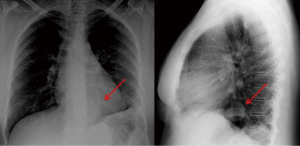
Complete abdomen ultrasound and bone scans were negative. Conversely, a standard chest X-ray showed a 4 cm paracardiac round opacity associated with obliteration of the costophrenic angle and ascension of the left hemidiaphragm (Figure 1). A computed tomography (CT) scan confirmed the presence of a 4.5 cm paracardiac solid mass (Figure 2A,B) associated with some left-sided pleural nodules of 2 cm in maximum diameter (Figure 2C). A fluorine-18-deoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan showed no pathologic uptake.

According to the pathological stage (IIIC) and taking into account the low sensitivity of 18F-FDG PET/CT in the evaluation and staging of invasive lobular carcinoma (4), this radiological presentation could be suspicious for metastatic dissemination.
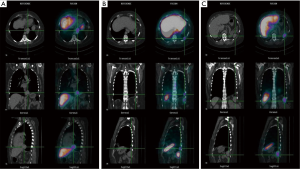
After inconclusive imaging tools, patient was eligible to receive a diagnostic thoracoscopy. Interestingly, a more accurate collection of her medical history allowed discovering that she underwent splenectomy and left nephrectomy consecutive to a severe thoraco-abdominal trauma, 30 years ago. In the context of a multidisciplinary board, we reviewed all available imaging and also a misleading solid mass in spleen bed was detected (Figure 2D). After case revision and according to patient’s medical history, a diagnosis of TS was supposed. Surgical planning was stopped and a technetium-99-labeled nanocolloid scan was performed to confirm our diagnostic hypothesis. Scintigraphy was performed with antero-posterior planar images and single photon emission computed tomography (SPECT) acquisition of the thorax and upper abdominal region. The fused SPECT-CT images demonstrated that all nodules suspected for malignancies corresponded to multiple foci of radioisotope reticulo-endothelial specific uptake, confirming the diagnosis of thoracic and abdominal splenosis (Figure 3). Therefore, after excluding the malignant origin and avoiding more aggressive diagnostic procedures, patient underwent adjuvant treatments for breast cancer.
Discussion
To date, more than seventy cases of TS have been reported and, to the best of our knowledge, only three cases in cancer patients (colon, melanoma and lung) (5-7).
This entity has to be differentiated from other benign (e.g., vascular lesions, amarthomas, infectious lesions, fibrosis, rheumatologic diseases, round atelectasis) or malignant conditions, such as pleural mesothelioma, lymphomas, thymoma and metastases from other primary malignancies. Therefore, the diagnosis can be very difficult or misleading, particularly in presence of a patient with a personal history of cancer, and it can be reached after unnecessary invasive approaches. In fact, more than half of all cases have been diagnosed after invasive procedures, such as fine-needle aspiration (FNA) or fine-needle aspiration biopsy (FNAB), and video-assisted thoracoscopic surgery (VATS) or thoracotomy (8). Notably, these invasive approaches are not free of complications, such as bleeding. Furthermore, the pathologic diagnosis can be inconclusive because of scarcity of cytological/histological samples or confused with lymphoproliferative disorders (3).
In presence of solitary or multiple left-sided pleural nodules in patients with a history of thoraco-abdominal trauma and splenectomy, a prospective diagnosis of TS should be considered and finally confirmed using non-invasive diagnostic tools, such as CT, magnetic resonance imaging (MRI) and specific radionuclide scans.
TS, revealed by CT imaging, consists of solitary or multiple non-specific enhanced round pleural nodules in the left hemitorax up to 3 cm in maximum diameter, although major sizes can be reached (9). This imaging technique is not able to narrow the diagnosis, as well as standard MRI. A better accuracy has been demonstrated by using femuroxides, super-paramagnetic iron oxide-enhanced MRI agents that have shown a biodistribution to phagocytic reticuloendothelial system, rendering them tissue-specific contrast agents (10).
The mainstay in the diagnosis of TS remains scintigraphy by using specific radionuclides, such as 99mTc sulfur colloid, indium 111-labeled platelet, 99mTc heat-damaged erythrocyte, or the 99mTc white blood cell (8).
Scintigraphy with radiocolloids is extensively used in clinical practice because of the large availability of this radiotracer, commonly used to detect sentinel lymph node in breast cancer (11) and melanoma (12). It is employed in hepatosplenic disorders, due to its specific uptake in the reticulo-endothelial system by phagocytic Kuppfer’s cells.
However, scintigraphy using 99mTc heat-damaged erythrocyte (RBC) or indium 111-labeled platelet appears to be superior to that with radiocolloids in terms of accuracy, due to a reduced uptake by normal liver tissue and a higher splenic sequestration and phagocytosis. Different studies demonstrated that 99mTc-RBC scan is more sensitive and specific when compared with 99mTc sulfur colloid one in detecting splenosis, with a difference in terms of sensitivity reaching 32% (13,14). This behavior renders these radionuclide scans the current diagnostic methods of choice.
Furthermore, scintigraphy can be performed with antero-posterior planar images and SPECT acquisition of the thorax and upper abdominal region. The fused SPECT-CT images show the specific uptake into reticulo-endothelial tissue and they allow localizing the exact anatomic sites of the ectopic spleen implants (15).
To the best of our knowledge, this is the first case report of TS in a patient with breast cancer. In our opinion, it warrants some considerations.
It is noteworthy that a correct differential diagnosis was very difficult in our case. In particular, a misleading interpretation of radiological images could be related to the recent diagnosis of breast cancer and its pathological staging. 18F-FDG PET-CT scan was negative but inconclusive due to its low sensitivity in the evaluation of invasive lobular carcinoma of the breast. We believe that the accurate collection of patient’s medical history and the collaboration within a multidisciplinary team marked a turning point in the diagnostic-therapeutic process.
In fact, both case discussion and imaging review at multidisciplinary board involving oncologist, radiologist, thoracic surgeon and nuclear physician led to suspect a diagnosis of TS and to recommend a diagnostic algorithm including specific radionuclide scan before proceeding with a surgical tool.
The choice to use nanocolloid radionuclide derived from its large availability and from long experience of our nuclear physicians.
By using this approach we had early a correct diagnosis of TS, avoiding more aggressive procedures that could cause unnecessary complications and delay adjuvant therapies that patient needed.
In conclusion, the diagnosis of TS has to be suspected in presence of left-sided pleural nodules in patients with a history of thoraco-abdominal trauma and splenectomy and it should be always reached by using and integrating non-invasive tools, mainly specific radionuclide scans.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: An informed consent form could not be obtained because the patient was dead before the manuscript was submitted. An absolute confidentiality of the patient’s vital information was maintained for ethical purposes.
References
- Normand JP, Rioux M, Dumont M, et al. Thoracic splenosis after blunt trauma: frequency and imaging findings. AJR Am J Roentgenol 1993;161:739-41. [Crossref] [PubMed]
- Yammine JN, Yatim A, Barbari A. Radionuclide imaging in thoracic splenosis and a review of the literature. Clin Nucl Med 2003;28:121-3. [Crossref] [PubMed]
- Thourani VH, Sharma J, Duarte IG, Miller JI Jr. Intrathoracic splenosis. Ann Thorac Surg 2005;80:1934-6. [Crossref] [PubMed]
- Groheux D, Giacchetti S, Moretti JL, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging 2011;38:426-35. [Crossref] [PubMed]
- Marchiori E, Rodrigues RS, Reis MC, et al. Pleural nodules in a patient with a colonic tumour. Thorax 2014;69:395, 398.
- Biron-Schneider AC, Clemenson A, Tiffet O, et al. Thoracic splenosis mimicking pleural and pulmonary metastasis. Ann Pathol 2010;30:382-5. [Crossref] [PubMed]
- Artinian MA, Gilliam JI. CT of intrathoracic splenosis in the presence of bronchogenic carcinoma. J Comput Assist Tomogr 1993;17:827-8. [Crossref] [PubMed]
- Malik UF, Martin MR, Patel R, et al. Parenchymal thoracic splenosis: history and nuclear imaging without invasive procedures may provide diagnosis. J Clin Med Res 2010;2:180-4. [PubMed]
- O'Connor JV, Brown CC, Thomas JK, et al. Thoracic splenosis. Ann Thorac Surg 1998;66:552-3. [Crossref] [PubMed]
- Prosch H, Oschatz E, Pertusini E, et al. Diagnosis of thoracic splenosis by ferumoxides-enhanced magnetic resonance imaging. J Thorac Imaging 2006;21:235-7. [Crossref] [PubMed]
- Yararbas U, Argon AM, Yeniay L, et al. The effect of radiocolloid preference on major parameters in sentinel lymph node biopsy practice in breast cancer. Nucl Med Biol 2010;37:805-10. [Crossref] [PubMed]
- Uhara H, Yamazaki N, Takata M, et al. Applicability of radiocolloids, blue dyes and fluorescent indocyanine green to sentinel node biopsy in melanoma. J Dermatol 2012;39:336-8. [Crossref] [PubMed]
- Hagan I, Hopkins R, Lyburn I. Superior demonstration of splenosis by heat-denatured Tc-99m red blood cell scintigraphy compared with Tc-99m sulfur colloid scintigraphy. Clin Nucl Med 2006;31:463-6. [Crossref] [PubMed]
- Gunes I, Yilmazlar T, Sarikaya I, et al. Scintigraphic detection of splenosis: superiority of tomographic selective spleen scintigraphy. Clin Radiol 1994;49:115-7. [Crossref] [PubMed]
- Crivellaro C, Cabrini G, Gay E, et al. Intrathoracic splenosis: evaluation by 99mTc-labelled heat-denatured erythrocyte SPECT/CT. Eur J Nucl Med Mol Imaging 2011;38:412. [Crossref] [PubMed]