
A test of miR-128-3p and miR-33a-5p in serum exosome as biomarkers for auxiliary diagnosis of non-small cell lung cancer
Highlight box
Key findings
• MiR-128-3p and miR-33a-5p demonstrated advantages in diagnosis of NSCLC, and the combined detection of miR-128-3p and miR-33a-5p showed higher sensitivity.
What is known and what is new?
• MiR-128-3p and miR-33a-5p were significantly down-regulated in NSCLC serum exosomes compared with paired normal tissues.
• Serum exosomes miR-128-3p and miR-33a-5p showed good performance in NSCLC screening with excellent sensitivity and specificity.
What is the implication, and what should change now?
• Both miR-128-3p and miR-33a-5p may be potential novel biomarkers for distinguishing NSCLC patients and healthy individuals. Now, what we need is to build high-quality NSCLC prediction models including miRNA to improve the diagnosis rate of NSCLC. And, testing the function of miR-128-3p and miR-33a-5p in NSCLC is an essential step.
Introduction
Lung cancer is one of the most common malignancies and remains the leading cause of cancer-related deaths worldwide (1-3). According to global statistics published in The Lancet, there were 3.3 million new lung cancer cases and 1.7 million deaths in 2015, suggesting lung cancer is a major threat to human health in the 21st century, while non-small cell lung cancer (NSCLC) is the most common type of lung cancer with the highest mortality rate (4). The stage at diagnosis is a major determinant of lung cancer prognosis, and resection is the primary mode of treatment for lung cancer. However, there is still a lack of specific tumor markers for NSCLC screening. Although related studies have confirmed that carcinoembryonic antigen, cancer antigen 125 and cytokeratin fragment 21-1 are serum antigenic biomarkers associated with lung cancer. And only by incorporating some clinical variables (nodule size, smoking history, age, etc.) can these biomarkers improve the diagnostic accuracy of lung cancer and reduce the invasive diagnosis and treatment of benign nodules, but also delay the treatment of malignant nodules to a certain extent, and the clinical application is limited. Besides, the value of circulating tumor DNA (ctDNA) as a biomarker of advanced tumor has been well established. However, its role in lung cancer screening and auxiliary diagnosis is still uncertain (5-8). In addition, conventional diagnostic methods have certain disadvantages that limit their application in the screening of malignant tumors. For example, imaging examinations are less specific, have certain requirements on lesion size, and are not easy to perform. Although histopathology is the gold standard for tumor diagnosis, it requires tissue sampling and accurate lesion locating prior to sampling (9,10). Therefore, it is of great significance to develop more efficient auxiliary diagnostic methods for NSCLC to improve prognosis, prolong survival, and increase the quality of life in NSCLC patients.
Liquid biopsy is a promising new technology for the early screening of malignant tumors by obtaining tumor information through non-invasive blood sampling to assist the diagnosis and treatment of malignant tumors (11). MicroRNAs (miRNAs) are a family of 21–25 nucleotide-long non-coding small RNA molecules that play key roles in important life processes such as embryonic development, cell cycle regulation, proliferation/differentiation, and apoptosis. There is increasing evidence that there are abnormalities in the expressions of a variety of miRNAs in human tumors, suggesting that miRNAs may play important roles in the pathogenesis of malignant tumors by regulating the expressions of oncogenes or tumor suppressor genes. A recent study on miRNA subtypes revealed the potential of blood miRNAs in indicating complex diseases and thus miRNAs may be novel clinical parameters for liquid biopsy (12). Exosomes are 40–100 nm membrane vesicles secreted by most cell types, which exist in serum, urine, saliva and other body fluids. Exosomes can carry RNA to shuttle freely between cells and tissues, connect the communication network between cells, and widely participate in many biological processes such as immune response, antigen presentation, cell differentiation, tumor growth and invasion (13-15). Relevant studies have shown that lung cancer-related exosomes can affect the occurrence and progression of lung cancer by regulating the physiological functions of surrounding tissue cells and microenvironment, and are considered to be an important part of lung cancer fluid biopsy. For example, exosomes regulate the function of immune cells such as T lymphocytes, dendritic cells, and natural killer cells by transferring immunosuppressive factors such as exocrine miRNAs. Furthermore, it affects the occurrence and development of lung cancer: the high level of miR-660-5p in exocrine promotes the progression of NSCLC by targeting KLF9, miR-21/29a initiates the growth and metastasis of lung cancer by activating TLR7 and TLR8 on immune cells, and the communication between lung cancer cells and CD4+ lymphocytes through exosome mir-214, which effectively reduces the expression of PTEN and promotes the expansion of regulatory T cells and tumor growth (13,16-18). Therefore, with the advancement of RNA sampling and detection technology, miRNA in exosome is relatively stable and can be effectively recovered in biological fluid, making people increasing interests in using miRNA as biomarkers for NSCLC screening or for monitoring tumor recurrence or metastasis after surgery (19).
Relevant studies have shown that miR-128-3p and miR-33a-5p, as tumor suppressor genes, can affect the occurrence, invasion, and metastasis of gastric cancer, esophageal cancer, breast cancer, colorectal cancer, melanomas, and other cancers, through different signaling pathways (20-23). In lung cancer, miR-33a-5p has been reported to inhibit the proliferation and invasion of lung cancer cells. In addition, the expression of miR-128-3p in lung cancer was significantly downregulated, and the repair of miR-128-3p in vivo could significantly suppress the tumorigenesis of A549 cells in nude mice models and inhibit angiogenesis and lymphangiogenesis in tumour xenografts (24-26). More importantly, the reason why we pay attention to the possibility that miR-128-3p and miR-33a-5p can be used as biomarkers for NSCLC screening is that relevant studies have shown that these two miRNAs are significantly down-regulated in NSCLC tissues than in matched normal tissues, indicating that their content is associated with lung cancer cells (27). However, due to the limitations of lung tissue collection, the clinical application of these two miRNAs in the diagnosis of lung cancer is very difficult. Compared with the acquisition of lung tissue samples, blood samples are easier to collect. Therefore, in this present study, by detecting the expressions of miR-128-3p and miR-33a-5p in the serum exosome of NSCLC patients and healthy volunteers using quantitative real-time polymerase chain reaction (qRT-PCR), we explored the relationships of these two miRNAs with the pathogenesis of NSCLC and assessed the sensitivity and specificity of different diagnostic models, with a view to providing potential and reliable biomarkers for early diagnosis and monitoring of NSCLC. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-398/rc).
Methods
Subjects
The inclusion criteria for the case group were as follows: (I) inpatients scheduled for surgery; (II) computed tomography (CT) of the lungs using a 64-slice spiral CT scanner (Philips, Amsterdam, the Netherlands) showed peripheral pulmonary nodules sized 0.5–5.0 cm, which were highly suspected as lung cancer; and (III) postoperative pathological results confirmed the diagnosis of NSCLC. The inclusion criteria for the control group included the following: (I) no pulmonary nodule was seen on CT of the lungs using a 64-slice spiral CT scanner (Philips); and (II) no history of malignancy. All subjects were treatment-naive and had not received neoadjuvant therapy such as chemotherapy or radiotherapy. In addition, all participants must have complete medical records and imaging examination information.
The exclusion criteria included the following: (I) patients with severe coronary heart disease, severe bronchial asthma, cardiomyopathy, malignant tumors other than NSCLC, allergic diseases, severe liver and kidney dysfunction, hematopoietic system diseases, neurological diseases, psychiatric diseases, autoimmune diseases, active tuberculosis, and/or other diseases; (II) individuals with communication problems; and (III) pregnant and lactating women.
A total of 20 patients with lung nodules who were highly suspected of having lung cancer were recruited. Two cases were excluded, with one patient failing to undergo surgery and the other patient presenting with a benign lesion. Thus, the case group included 18 patients who received surgical treatment and were pathologically confirmed with NSCLC (postoperative pathological diagnosis is the gold standard for the diagnosis of lung cancer). A total of 18 healthy volunteers were recruited as the control group. All participants were recruited from our center from September 1, 2022 to December 30, 2022. Among these 36 subjects, there were 20 females and 16 males, with an average age of 60.94±6.08 years (Figure 1).
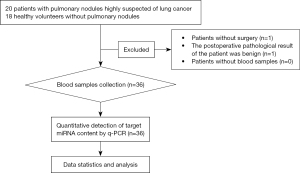
Before the initiation of the study, all subjects were evaluated and screened by the researchers to determine whether they were eligible for the study and to finalize the grouping based on the tests received and the postoperative pathological results. The subject data were registered in the thoracic surgeons’ offices. The clinical data were extracted from the patients’ medical records.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Changhai Hospital (No. 2020021) and informed consent was taken from all the participants.
Reagents and equipment
Reagents and equipment used included the following: serum/plasma miRNA extraction kits and primers (Shanghai Puxi Biotech, Shanghai, China); grinder (MP Bio, Santa Ana, CA, USA); spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA); refrigerated centrifuge (Beckman Coulter, Brea, CA, USA); Ultra-highspeed centrifuge (Thermo Fisher Scientific); gel scanner (Bio-Rad, Hercules, CA, USA); and real-time PCR machine (Bio-Rad).
Collection of serum exosomes
Venous blood (10 mL) was collected 1 day before surgery using ordinary vacuum blood-collection tubes. After 10–60 min of clotting at 4 ℃, the blood samples were centrifuged at 300×g for 10 min. The upper layer of the serum (yellow) was carefully transferred to a new 1.5 mL centrifuge tube and centrifuged at 2,000×g for 10 min at 4 ℃. Take the new supernatant and continue to increase the centrifugal force to 10,000×g for 30 min, and then the cell fragments were removed. After treatment, the supernatant was centrifuged for 70 min with 100,000×g centrifugal force to obtain crude exocrine sediment (containing a small amount of foreign protein). Use phosphate buffer solution (PBS) to resuspend the exocrine precipitate, and then 100,000×g again, centrifuge for 70 min to obtain pure exocrine, and stored at −80 ℃ until use. None of the patients experienced significant adverse effects after blood collection.
Measurements
To determine of the expression levels of miR-128-3p and miR-33a-5p, total RNA was extracted from the treated samples using the RNA extraction kit (9109, TAKARA, Beijing, China) and reversed into cDNA using the reverse transcription kit (Bio-Rad, CFX Connect). The experimental procedures were conducted in strict accordance with kit instructions. The miR-128-3p, miR-33a-5p, and their internal reference U6 were amplified by qRT-PCR instrument. The detailed primer sequences are shown in Table 1. The qRT-PCR reaction system included the following: 2× iTaqTM universal SYBR Green supermix (5 µL), forward and reverse primers (1 µL), and DNA template (2 µL). In order to reduce experimental error, all experiments were performed in triplicate wells. The relative expressions of the serum exosome miR-128-3p and miR-33a-5p were calculated using the Bio-Rad CFX Manager software. Laboratory staffs were blinded to the patient information corresponding to the blood samples throughout the tests, and they were only responsible for sample testing and data reporting.
Table 1
| Primer name | Sequence (5' to 3') |
|---|---|
| hsa-miR-33a-5p -F | GCGCGTGCATTGTAGTTGC |
| hsa-miR-128-3p -F | GCGCTCACAGTGAACCGGT |
| Universal primer R | CGA GGAAG AAGA CGG AAGAAT |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
Statistical analysis and visualization
All data were analyzed using the SPSS 25.0 software package and visualized using the Graphpad Prism 9 software. Measurement data are expressed as mean ± standard deviation, and count data are expressed as frequency or rate (%). Inter-group comparisons were based on t-tests. The Pearson method was used to analyze the correlation between the expression levels of miR-128-3p and miR-33a-5p in the serum exosome. Application of logistics regression analysis method to establish combined diagnosis model. The receiver operating characteristic (ROC) curves were drawn using the IBM SPSS Statistics 26 software package, and the area under the ROC curves (AUC), sensitivity, and specificity of different indicators in diagnosing NSCLC were analyzed, and the cut-off value was the threshold to determine the sensitivity and specificity. The R studio software was used to analyze the difference in AUCs. A P value <0.05 was considered statistically significant. Patients with missing or inconclusive data were excluded from the final analysis.
Results
Clinical features
The clinical features of the participants are shown in Table 2. The median age was 59.5 years (range, 50–72 years), and the median age of patients in the case group was 58.5 years (range, 53–72 years). There were 9 males (50%) and 9 females (50%) in the case group, with 5 patients (27.8%) presenting with a history of smoking. According to the 8th edition of AJCC/UICC staging criteria, there were 8 cases with stage IA tumors (44.4%), 6 cases (33.3%) with stage IB, 3 cases (16.7%) with stage IIB, and 1 case (5.6%) with stage IIIA tumor. The maximum tumor diameter ranged from 0.8 to 5.7 cm (median: 3.1 cm). All enrolled patients were naive to neoadjuvant radiochemotherapy or traditional Chinese medicine treatments. The median age in the control group was 61.5 years (range, 50–69 years). No pulmonary nodule was detected by chest CT in the control group.
Table 2
| Variables | Control (n=18) | Lung cancer (n=18) |
|---|---|---|
| Age (years) | ||
| Median [range] | 61.5 [50–69] | 58.5 [53–72] |
| Gender, n (%) | ||
| Female | 11 (61.1) | 9 (50.0) |
| Male | 7 (38.9) | 9 (50.0) |
| Tobacco and alcohol use, n (%) | ||
| Both | 2 (11.1) | 3 (16.7) |
| Tobacco only | 3 (16.7) | 2 (11.1) |
| Alcohol only | 4 (22.2) | 2 (11.1) |
| None | 9 (50.0) | 11 (61.1) |
| Family history of cancer, n (%) | ||
| Yes | 1 (5.6) | 2 (11.1) |
| No | 17 (94.4) | 16 (88.9) |
| Tumor location, n (%) | ||
| Left | 8 (44.4) | |
| Right | 10 (55.6) | |
| Maximum tumor diameter (cm) | ||
| Median [range] | 3.1 [0.8–5.7] | |
| Lymph node stage, n (%) | ||
| pN0 | 16 (88.9) | |
| pN1 | 1 (5.6) | |
| pN2–pN3 | 1 (5.6) | |
| TNM stage, n (%) | ||
| 0–I | 14 (77.8) | |
| II | 3 (16.7) | |
| III | 1 (5.6) | |
TNM, tumor-node-metastasis
Differential expression levels of miR-128-3p and miR-33a-5p levels in serum exosomes
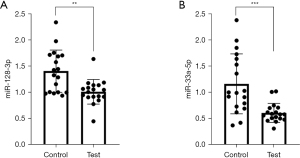
A comparison of the differential expression levels of miR-128-3p and miR-33a-5p in the serum exosomes showed that the expression of miR-128-3p and miR-33a-5p were significantly lower in the case group than in the control group (P<0.05; Figure 2).
The correlation between miR-128-3p and miR-33a-5p expression levels in subjects
The mean values of three measurements for miR-128-3p and miR-33a-5p in each sample were taken as the observation data, and the correlation between these two genotypes was analyzed. These mean values were normally distributed, and the results of Pearson correlation analysis are shown in Table 3. The correlation coefficient between miR-128-3p and miR-33a-5p in the serum was 0.848 (r=0.848, 2-tailed), suggesting that the levels of miR-128-3p and miR-33a-5p were positively correlated at the level of 0.01.
Table 3
| miR-128-3p | miR-33a-5p | |
|---|---|---|
| miR-128-3p | ||
| Pearson correlation | 1 | 0.848** |
| Sig. (2-tailed) | – | 0.000 |
| N | 36 | 36 |
| miR-33a-5p | – | |
| Pearson correlation | 0.848** | 1 |
| Sig. (2-tailed) | 0.000 | – |
| N | 36 | 36 |
**, P<0.01.
miR-128-3p and miR-33a-5p are effective biomarkers for distinguishing non-small cell NSCLC patients from healthy individuals
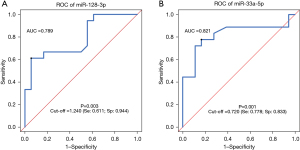
ROC curves were drawn to evaluate the ability of miR-128-3p or miR-33a-5p to distinguish between NSCLC patients and healthy controls. The AUC value of miR-128-3p alone in distinguishing case group and control group was 0.789 (95% CI: 0.637–0.940; sensitivity: 61.1%; specificity: 94.4%; P=0.003), with a cut-off value of 1.240 (Figure 3A). The AUC value of miR-33a-5p alone in distinguishing case group and control group was 0.821 (95% CI: 0.668–0.974; sensitivity: 77.8%; specificity: 83.3%; P=0.001), with a cut-off value of 0.720 (Figure 3B). Thus, both miR-128-3p and miR-33a-5p could be used to distinguish NSCLC patients and healthy controls, with miR-128-3p showing a higher specificity (94.4%) and miR-33a-5p showing a higher sensitivity (77.8%).
The combined use of miR-128-3p and miR-33a-5p as a biomarker for distinguishing NSCLC patients from healthy individuals
Based on a logistics model, the risk score of the combination of miR-128-3p and miR-33a-5p was calculated using the following formula: risk score = miR-128-3p × (−2.223) + miR-33a+5p × (−3.678) + 5.428. This risk score model was used to make ROC curve to evaluate the ability of combining miR-128-3p and miR-33a+5p to predict NSCLC. We called this model “model-combination A”. The AUC calculated according to the risk score was 0.855 (95% CI: 0.719–0.991; P<0.001), and the optimal cut-off value was 0.034. A diagnosis of NSCLC was made if the risk score was larger than 0.034, with the sensitivity being 83.3% and the specificity being 88.9% at this point (Figure 4). The ROC curves of serum exosome miR-128-3p, miR-33a-5p, and model-combination A were compared using the R studio software. The AUCs of miR-128-3p and miR-33a-5p showed no significant differences to that of model-combination A (P=0.3664 and P=0.2142, respectively; Table 4), suggesting that the performance of the combined detection of serum exosome miRNAs was not superior to that of serum exosome miR-128-3p alone nor miR-33a-5p alone.
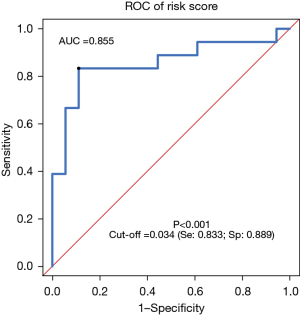
Table 4
| Model name | Model-miR-33a-5p | Model-miR-128-3p | Model-combination A | Model- combination B |
|---|---|---|---|---|
| Model-miR-33a-5p | – | |||
| Model-miR-128-3p | 0.7186 | – | ||
| Model-combination A | 0.2142 | 0.3664 | – | |
| Model- combination B | 0.776 | 0.5814 | 0.583 | – |
In pursuit of higher sensitivity, 1.240 was selected as the diagnostic criterion for miR-128-3p and 0.720 was selected for miR-33a-5p. A diagnosis of NSCLC would be made if any of these two indicators decreased. Based on this criterion, the prediction model had a sensitivity of 0.833 (15/18) and a specificity of 0.833 (15/18) (Table 4) (referred to as model-combination B). Analysis using R studio software found no statistically significant difference between model-combination B and the previous three models (P=0.5814, P=0.776, and P=0.583, respectively; Table 4).
Discussion
Lung cancer is the malignant tumor with the highest incidence in the world today, which poses a serious threat to human health. If lung cancer can be treated with thoracoscopic resection at the time of discovery, lung cancer metastasis and mortality can be reduced (10,28). Therefore, it is of great significance to find suitable biomarkers for lung cancer screening and auxiliary diagnosis. Advances in liquid biopsy may greatly contribute to the auxiliary diagnosis and treatment of lung cancer. To date, liquid biopsy has been widely used in three clinical areas: (I) tumor detection and progression monitoring (29,30); (II) diagnosis of heart attack (31); and (III) prenatal diagnosis (32). In addition, it can be applied to cancer research in two main aspects: (I) detection and monitoring of circulating tumor cells (33); and (II) identification of cell-free tumor DNA (34). These applications of liquid biopsy in cancer research confirmed their applicability for clinical identification, long-term monitoring, and prognosis prediction of multiple tumor types (e.g., lung cancer, liver cancer, and colorectal cancer) (30,33,35). Relevant studies have found that miR-21, miR-30d, miR-451, miR-10a, miR-30e-5p, and miR-128 are potential biomarkers for lung cancer diagnosis, suggesting that miRNAs may play a prominent role in clinical diagnosis and monitoring (35,36). Due to their great potential, exosomal miRNAs can be used as an excellent non-invasive tool for diagnosis, prognosis and prediction of treatment success or drug resistance in this pathology. However, there are still challenges to be achieved. Firstly, because of the heterogeneity in size (different subtypes of vesicles), standardized methodologies must be established for isolation, characterization and study of exosomes cargo. Secondly, identifying and validating the unique clinical value of each candidate miRNA is expensive and time-consuming (13,35,37-39). Therefore, the mature and stable testing process is indispensable, and in order to lower the economic burden of patients, we selected only a single or a few miRNAs that affect the development and progression of NSCLC for detection and analysis.
In our present case-control study, the levels of miR-128-3p and miR-33a-5p in serum exosomes were measured in both NSCLC patients and healthy controls, and the value of miR-128-3p alone, miR-33a-5p alone, and the combination of miR-128-3p and miR-33a-5p as biomarkers for NSCLC screening was compared. The serum exosome levels of both miR-128-3p and miR-33a-5p were significantly lower in NSCLC patients than those in the healthy controls, and the miR-128-3p expression was positively correlated with miR-33a-5p expression. Both the detection of serum exosome miR-128-3p alone or the combined detection of miR-128-3p and miR-33a-5p can be used to distinguish NSCLC patients from healthy controls. The combined use of miR-128-3p and miR-33a-5p had the largest AUC and the highest sensitivity compared with either miR-128-3p alone or miR-33a-5p alone, however, the difference in AUC was not statistically significant, indicating that the combined detection of these two miRNAs was not superior to the detection of either miR-128-3p or miR-33a-5p alone. However, this study was limited by its sample size and subgroup analyses on NSCLC at different stages and with different degrees of differentiation were not performed. It is speculated that miR-128-3p or miR-33a-5p may become auxiliary indicators for determining the stage and degree of malignancy of NSCLC.
In summary, exosomes miR-128-3p and miR-33a-5p showed good performance for NSCLC screening, and both can be used as biomarkers for NSCLC screening, and the combined detection of exosomes miR-128-3p and miR-33a-5p has higher sensitivity. Therefore, these two miRNAs may be widely used in clinical screening and auxiliary diagnosis of NSCLC in the future, and it may be more cost-effective to detect a single miRNA for the economic benefit of patients. However, this study still has some limitations, and the clinical application of these two miRNAs still needs further research. Firstly, the sample size of this study is small, because of various reasons, only 36 patients can be included for data analysis, but the value of these biomarkers should be verified in studies with larger sample sizes, which is also what we will do in the future. Since stability determines whether a miRNA can be used as a biomarker (5), the stabilities of miR-128-3p and miR-33a-5p should be further verified and analyzed under various harsh conditions (e.g., radiotherapy, chemotherapy, and immunotherapy). Secondly, to avoid false positives, serum exosome expressions of miR-33a-5p and miR-128-3p in NSCLC patients should also be distinguished from those in benign lung diseases (e.g., pneumonia and tuberculosis). Besides, a more stable and accurate NSCLC screening model should be established by integrating general features including tumor indexes, gender, age, family history, smoking history, and occupational history. It is also necessary to compare the levels of miR-128-3p and miR33a-5p in different NSCLC stages or in different NSCLC subgroups, and to establish a prognosis prediction model in combination with tumor indicators, pathological stages, tissue differentiation degree and other factors, so as to strengthen postoperative monitoring, management and timely intervention of NSCLC patients. However, more importantly, we have not yet studied the mechanism of exocrine miR-128-3p and miR-33a-5p regulating the occurrence and development of NSCLC, which limits the clinical value of miRNA. Therefore, in the follow-up research, we will study the mechanism of the effects of these two miRNAs on NSCLC, in order to find the key targets to regulate different biological processes such as lung cancer growth, progression, invasion, angiogenesis, metastasis and drug resistance.
Conclusions
From this study, we learned that miR-128-3p and miR-33a-5p demonstrated advantages in the auxiliary diagnosis of NSCLC, and the combined detection of miR-33a-5p and miR-128-3p showed higher sensitivity. Both miR-128-3p and miR-33a-5p may be potential novel biomarkers for distinguishing NSCLC patients and healthy individuals. This discovery encourages the progress of miRNAs research related to NSCLC, not only as a new biomarker, but also may lead to the development of new pharmacological drugs to assist the treatment of NSCLC.
Acknowledgments
Funding: This work was supported by the Shanghai Society of Integrated Traditional Chinese and Western Medicine Community Medicine and Health Management Research Fund Project (No. SQ26).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-398/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-398/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-398/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-398/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of Changhai Hospital (No. 2020021) and informed consent was taken from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang N, Nan A, Chen L, et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer 2020;19:101. [Crossref] [PubMed]
- Nasim F, Sabath BF, Eapen GA. Lung Cancer. Med Clin North Am 2019;103:463-73. [Crossref] [PubMed]
- Oliver AL. Lung Cancer: Epidemiology and Screening. Surg Clin North Am 2022;102:335-44. [Crossref] [PubMed]
- Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. [Crossref] [PubMed]
- Seijo LM, Peled N, Ajona D, et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol 2019;14:343-57. [Crossref] [PubMed]
- Mazzone PJ, Wang XF, Han X, et al. Evaluation of a Serum Lung Cancer Biomarker Panel. Biomark Insights 2018;13:1177271917751608. [Crossref] [PubMed]
- Merker JD, Oxnard GR, Compton C, et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 2018;36:1631-41. [Crossref] [PubMed]
- Giroux Leprieur E, Herbretau G, Dumenil C, et al. Circulating tumor DNA evaluated by Next-Generation Sequencing is predictive of tumor response and prolonged clinical benefit with nivolumab in advanced non-small cell lung cancer. Oncoimmunology 2018;7:e1424675. [Crossref] [PubMed]
- Zhang YH, Jin M, Li J, et al. Identifying circulating miRNA biomarkers for early diagnosis and monitoring of lung cancer. Biochim Biophys Acta Mol Basis Dis 2020;1866:165847. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Chudasama D, Katopodis P, Stone N, et al. Liquid Biopsies in Lung Cancer: Four Emerging Technologies and Potential Clinical Applications. Cancers (Basel) 2019;11:331. [Crossref] [PubMed]
- Yu H, Guan Z, Cuk K, et al. Circulating microRNA biomarkers for lung cancer detection in Western populations. Cancer Med 2018;7:4849-62. [Crossref] [PubMed]
- Duréndez-Sáez E, Torres-Martinez S, Calabuig-Fariñas S, et al. Exosomal microRNAs in non-small cell lung cancer. Transl Cancer Res 2021;10:3128-39. [Crossref] [PubMed]
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem 2019;88:487-514. [Crossref] [PubMed]
- Tang Z, Li D, Hou S, et al. The cancer exosomes: Clinical implications, applications and challenges. Int J Cancer 2020;146:2946-59. [Crossref] [PubMed]
- Li MY, Liu LZ, Dong M. Progress on pivotal role and application of exosome in lung cancer carcinogenesis, diagnosis, therapy and prognosis. Mol Cancer 2021;20:22. [Crossref] [PubMed]
- Jiang C, Zhang N, Hu X, et al. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol Cancer 2021;20:117. [Crossref] [PubMed]
- Qi Y, Zha W, Zhang W. Exosomal miR-660-5p promotes tumor growth and metastasis in non-small cell lung cancer. J BUON 2019;24:599-607. [PubMed]
- Yang X, Su W, Chen X, et al. Validation of a serum 4-microRNA signature for the detection of lung cancer. Transl Lung Cancer Res 2019;8:636-48. [Crossref] [PubMed]
- Song Q, Liu H, Li C, et al. miR-33a-5p inhibits the progression of esophageal cancer through the DKK1-mediated Wnt/β-catenin pathway. Aging (Albany NY) 2021;13:20481-94. [Crossref] [PubMed]
- Zhao J, Li D, Fang L. MiR-128-3p suppresses breast cancer cellular progression via targeting LIMK1. Biomed Pharmacother 2019;115:108947. [Crossref] [PubMed]
- Zhang ZR, Yang N. MiR-33a-5p inhibits the growth and metastasis of melanoma cells by targeting SNAI2. Neoplasma 2020;67:813-24. [Crossref] [PubMed]
- Yao J, Wang C, Dong X, et al. lncRNA SNHG22 sponges miR-128-3p to promote the progression of colorectal cancer by upregulating E2F3. Int J Oncol 2021;59:71. [Crossref] [PubMed]
- Pan J, Fang S, Tian H, et al. lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer 2020;19:9. [Crossref] [PubMed]
- Frixa T, Sacconi A, Cioce M, et al. MicroRNA-128-3p-mediated depletion of Drosha promotes lung cancer cell migration. Carcinogenesis 2018;39:293-304. [Crossref] [PubMed]
- Pan J, Zhou C, Zhao X, et al. A two-miRNA signature (miR-33a-5p and miR-128-3p) in whole blood as potential biomarker for early diagnosis of lung cancer. Sci Rep 2018;8:16699. [Crossref] [PubMed]
- Yang J, Li J, Le Y, et al. PFKL/miR-128 axis regulates glycolysis by inhibiting AKT phosphorylation and predicts poor survival in lung cancer. Am J Cancer Res 2016;6:473-85. [PubMed]
- Schabath MB, Cote ML. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol Biomarkers Prev 2019;28:1563-79. [Crossref] [PubMed]
- Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013;10:472-84. [Crossref] [PubMed]
- Wang Y, Yang L, Bao H, et al. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med 2021;18:e1003741. [Crossref] [PubMed]
- Bilbija D, Elmabsout AA, Sagave J, et al. Expression of retinoic acid target genes in coronary artery disease. Int J Mol Med 2014;33:677-86. [Crossref] [PubMed]
- Cai X, Janku F, Zhan Q, et al. Accessing Genetic Information with Liquid Biopsies. Trends Genet 2015;31:564-75. [Crossref] [PubMed]
- von Felden J, Garcia-Lezana T, Schulze K, et al. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut 2020;69:2025-34. [Crossref] [PubMed]
- Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget 2016;7:48832-41. [Crossref] [PubMed]
- Iqbal MA, Arora S, Prakasam G, et al. MicroRNA in lung cancer: role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med 2019;70:3-20. [Crossref] [PubMed]
- Tuo L, Chu X, Sha S, et al. MicroRNA and Lung Cancer: A Mini Review. Zhongguo Fei Ai Za Zhi 2018;21:727-30. [PubMed]
- Kim YH, Lee WK, Lee EB, et al. Combined Effect of Metastasis-Related MicroRNA, miR-34 and miR-124 Family, Methylation on Prognosis of Non-Small-Cell Lung Cancer. Clin Lung Cancer 2017;18:e13-20. [Crossref] [PubMed]
- Zhong S, Golpon H, Zardo P, et al. miRNAs in lung cancer. A systematic review identifies predictive and prognostic miRNA candidates for precision medicine in lung cancer. Transl Res 2021;230:164-96. [Crossref] [PubMed]
- Wu KL, Tsai YM, Lien CT, et al. The Roles of MicroRNA in Lung Cancer. Int J Mol Sci 2019;20:1611. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


