
Nutritional status in patients with active pulmonary tuberculosis and new nutritional risk screening model for active tuberculosis: a national, multicenter, cross-sectional study in China
Highlight box
Key findings
• Active TB patients are shown to have severe malnutrition status when screened by the NRS 2002 and GLIM criteria. A new screening model is recommended for PTB patients as it is closer to TB characteristics.
What is known, and what is new?
• TB transmission and progress are closely associated with and driven by poor nutrition status. Nutrition imbalance is likely to result in poor TB control. However, so far, no published studies have explored the best method of nutritional diagnosis for active TB.
• The present study provides the first evidence for malnutrition and establishes a new nutritional screening model for active TB to help optimize the outcomes of PTB.
What is the implication, and what should change now?
• We recommend that our newly established model is used to screen nutritional risk for TB patients instead of NRS 2002, as it has been adapted to TB clinical characteristics.
Introduction
Tuberculosis (TB) remains one of the leading infectious diseases worldwide, with an estimated 10.6 million new cases in 2021, according to the updated World Health Organization (WHO) report (1). It has been reported that undernourishment, smoking, drinking, drug addiction, and poverty are closely associated with TB occurrence (2). During the onset and progression of TB, nutrition consumption exists in all stages, which is regulated by host immune metabolic mechanisms such as interferon (IFN)-γ-dependent control or host-derived lipid metabolism in macrophages (3,4). Nutrition-associated metabolic or immunologic regulation plays an essential role in TB pathogenesis. Inhibited immunity against TB is associated with poor nutrition status (5).
TB transmission and progression are closely associated with and driven by poor nutrition status, which has been observed as one of the most common phenomena in clinics (6). Nutrition imbalance is likely to result in poor TB control, and poor nutrition can easily result in protein-energy and micronutrient deficiency, which can further increase the susceptibility to TB development (7). Clinicians have observed that many patients had malnutrition which might impact the treatment outcome. Nutritional supplementation can contribute to controlling TB and reducing mortality, as reported in India (8). Study has indicated that interventions with high-energy supplements such as a high-cholesterol diet, vitamins A and D, and multiple micronutrient supplements can help patients with active TB gain weight (9). However, there is insufficient evidence that sufficiently replenishing nutrition can improve the outcome of patients with active TB.
The Nutritional Risk Screening 2002 (NRS 2002) test evaluates the nutrition risk for the general population and all types of patients. Since active TB has been shown to have a close association with malnutrition, besides the factors listed in NRS 2002, there might be additional factors with a close association with malnutrition that could help to evaluate the nutritional status of patients with active TB. A previous study screening nutritional risk for TB patients showed that age, complications, body mass index (BMI), serum albumin, and length of hospital stay were higher in a group with nutritional risk than in a group without nutritional risk (10). A study in the general population showed that besides NRS 2002 risk factors, blood albumin score and C-reactive protein (CRP)/albumin ratio might be able to predict poor prognosis of in-patients (11). There are other nutritional diagnosis methods besides NRS 2002; the Global Leadership Initiative on Malnutrition (GLIM) test as the new malnutrition diagnostic criterion was collaboratively created by several major global nutritional societies in 2016 (12,13). Since TB patients have detailed characteristics such as drug resistance, extra-PTB, and co-infection with human immunodeficiency virus (HIV), TB patients need the most appropriate and accurate nutritional screening or diagnosis methods. However, so far, no published studies have explored the best method of nutritional diagnosis for active TB.
China has a high TB burden, ranking third in the severity of TB worldwide. To facilitate comprehension of the malnutrition status of active TB patients in China and attempt to build a screening model, especially for active PTB, a retrospective, cross-sectional, multicenter study in China with a large sample was conducted to investigate the malnutrition status for active PTB patients using the GLIM and NRS 2002 tools, and to analyze and evaluate the malnutrition factors to provide the most reliable evidence to construct a nutrition evaluation model for active TB. To our knowledge, few studies have evaluated and compared the utility of the NRS 2002 and GLIM for nutritional diagnosis of active PTB. Therefore, the present study aimed to provide the first evidence for malnutrition and further help to improve the outcome of patients with active PTB (8), especially for multidrug-resistant TB, which has represented the main challenge for TB control. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-623/rc).
Methods
Study design and patients
Patients who met the inclusion criteria were retrospectively enrolled in the study from 1 January 2020 to 31 December 2021. The study was designed as a multicenter, large cross-sectional, retrospective study. Patients were from 29 hospitals distributed in 28 cities from 17 provinces and 2 municipality-level cities, including the Shanghai Pulmonary Hospital from Shanghai in the east of China, Xi’an Chest Hospital from Shaanxi province in central China, Chongqing Public Health Center from Chongqing in the west of China, Hunan Chest Hospital from Hunan province in central China, Huzhou Center Hospital from Zhejiang province in the east of China, Fuzhou Pulmonary Hospital from Fujian province in southern China, Nanning No. 4 Hospital from Guangxi province in southern China, Jiangxi Chest Hospital from Jiangxi province in central China, Xinjiang Chest Hospital from Xinjiang autonomous region in the northwest of China, and several hospitals in the Liaoning, Heilongjiang, and Jilin provinces in the northeast of China.
The inclusion criteria were as follows: patients diagnosed with active PTB and had not been effectively treated or treated within one week, and completed information was retained for retrospective investigation.
The exclusion criteria were as follows: patients with an obscure diagnosis, no lesions in one or both lungs, or lack of essential information for the investigation, or patients with a positive mycobacteria culture positive but identified as non-tuberculous mycobacteria (NTM).
Ethical approval
This retrospective study conformed to the Declaration of Helsinki (as revised in 2013) for ethical principles for research and acquired approval from The Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (No. K20-431). All participating hospitals/institutions were informed and agreed the study. Since the study was retrospective, the ethics committee waived the requirement for written informed consent on the proviso that the privacy of patients enrolled was protected throughout the present study.
NRS 2002 and GLIM evaluation
All included patients were investigated for all the required information and underwent nutritional risk screening and evaluation by the NRS 2002 score standard (14,15). Patients evaluated with an NRS 2002 score ≥3 were judged as having nutritional risk, and those with <3 were judged as not having nutritional risk. The maximum total point score of NRS 2002 is 7 points, including 1 point from age. Included patients were also evaluated by the GLIM test, wherein the GLIM screening criteria were conducted according to the consensus report from the global clinical nutrition community (16). The GLIM criteria stipulated at least having 1 point from phenotypic criteria and 1 point from etiological criteria; the GLIM score was classified as “2” meaning having malnutrition, and “1” meaning not having malnutrition. The NRS 2002 score and GLIM standard are shown in Table S1.
Clinical investigation
Besides the factors included in NRS 2002 and GLIM tests, additional clinical factors and characteristics closely associated with PTB were investigated. Physicians and dieticians completed the investigation and evaluation; the content of the investigation included age, sex, BMI, smoking, alcohol consumption, final diagnosis, extra-PTB, the type of drug-resistant TB (DR-TB), the previous history of anti-TB treatment, Mycobacterium tuberculosis (MTB) etiological tests including MTB culture, acid-fast bacillus (AFB) smear results, molecular biological tests, comorbidities including diabetes mellitus (DM), tumor, transplantation, liver dysfunction, hepatitis, HIV, taking immunosuppressive agents, liver cirrhosis, anemia and so on, the severity of lung lesions including the lesion field, the number and diameter of the cavity on chest computed tomography (CT), clinical laboratory test results including lymphocyte count, CD4/CD8 in peripheral blood, blood lipid level including total cholesterol and triglyceride, and interferon-gamma release assays (IGRAs) results.
Patient diagnosis and classification of drug resistance and treatment history
The diagnosis standard for included patients with active PTB was according to the WHO guideline (17). DR-TB was classified into multidrug-resistant TB (MDR-TB), extent drug-resistant TB (XDR-TB), rifampicin-resistant-TB (RR-TB), and poly-drug-resistant TB (PDR-TB). MDR-TB was defined when the drug sensitivity test (DST) of a patient infected with MTB indicated at least resistance to both isoniazid (H) and rifampicin (RFP); XDR-TB was defined when the DST of a patient infected with MTB revealed resistance to at least isoniazid and rifampin and additional resistance to fluoroquinolones (FQs) or any second-line injectable agents (amikacin, kanamycin, or capreomycin) according to the 2016 WHO guidelines (18). RR-TB was defined when the DST of a patient infected with MTB revealed resistance to RFP; MDR-TB and XDR-TB were included by RR-TB. PDR-TB was defined when a patient infected with MTB exhibited resistance to 2 or more first-line anti-TB drugs, except resistance to RFP; PDR-TB was defined when a patient infected with MTB exhibited resistance to one first-line anti-TB drug, except resistance to RFP.
A newly diagnosed case was defined as a patient without previous anti-TB treatment history or having a treatment history for less than one month. A re-treated patient was defined as one with a previous anti-TB treatment history for over one month.
Bacteriological, radiological, and immunological tests for patients
Patients were tested by AFB smear, BACTEC MGIT 960 culture [MGIT 960; Becton, Dickinson, and Co. (BD), Franklin Lakes, NJ, USA], and DST, or L-J culture, identification, and DST, Xpert MTB/RIF (Cephid, Sunnyvale, CA, USA) in respiratory specimens for MTB etiology tests, chest CT for the radiological test, and IGRAs for immunological tests. Manufacturer protocols performed all examinations. Additionally, routine laboratory tests, including routine blood examination, blood fat, and peripheral flow cytometry test, were performed for patients according to the standard protocol.
Assignment ranks based on variables from patients investigated for further statistical analysis
The ranks were assigned for all included analysis variables, sex was categorized as female and male, age of patients was divided into five ranks: age <18, 18≤ age <40, 40≤ age <60, 60≤ age <80, and age ≥80 years; for BMI of patients were divided into three ranks: BMI ≥20.5, 18.5≤ BMI <20.5, BMI <18.5 kg/m2; serum albumin was divided into four ranks: serum albumin ≥35 g/L, ≤30 serum albumin <35 g/L, 25≤ serum <30 g/L, and serum albumin <25 g/L. The following statuses were divided into 2 ranks: drinking: no drinking, drinking; smoking: no smoking, smoking; presence of extra-PTB: no extra-PTB and extra-PTB; coexisting intestinal TB: no coexisting intestinal TB and coexisting intestinal TB; coexisting brain TB: no coexisting brain TB and coexisting brain TB; coexisting peritoneal TB: no coexisting peritoneal TB and coexisting peritoneal TB; coexisting lymph node TB: no coexisting lymph node TB and coexisting lymph node TB; coexisting bone TB: no coexisting bone TB and coexisting bone TB; coexisting pleural TB: having it or not; coexisting skin TB: having it or not; coexisting urinary system TB: having it or not; coexisting extra-PTB for ≥2 sites: having it or not; other categories included: newly diagnosed PTB and retreated PTB; AFB smear negative and AFB positive; MTB culture negative/positive; molecular test of MTB negative/positive; non-drug-resistant TB and drug-resistant TB; not MDR-TB and MDR-TB; not PDR-TB (except RR-TB) and PDR-TB; RR-TB (except MDR/XDR-TB) and not; XDR-TB and not; coexisting DM and not; coexisting chronic hepatitis and not; coexisting liver cirrhosis and not; coexisting transplantation and not; coexisting HIV infection and not; coexisting abdominal operation and not; taking immunosuppressive agents and not; coexistence of other chronic pulmonary diseases and not; coexisting dialysis and not; coexisting malignance and not; coexisting severe pneumonia and not; coexisting stoke and not; IGRAs negativity and positivity; CD4/CD8 <1 and ≥1; lesions ≥3 lung fields and not; pulmonary cavity lesions and not; lesion diameter in any cavity ≥3 cm and not. Lymphocyte absolute count in blood was divided into three ranks: >4×109/L, 1< cell count ≤4×109/L and ≤1×109/L; the status of change of weight loss: weight loss in 1 month >5%, weight loss in 2 months >5%, weight loss in 3 months >5%, and no noticeable change of weight loss; food intake in a week decreased by 25–50% compared to before, food intake in a week decreased by 51–75% compared to before, food intake in a week decreased by 76–100% compared to before, and no noticeable change; having edema result in inaccurate BMI and not. Having satisfied the GLIM criteria was scored as 2, and not having satisfied the GLIM criteria was scored as 1.
Statistical analysis
The data were first collected with SPSS 18.0 (IBM Corp., Armonk, NY, USA). The baseline data were compared between patients with NRS 2002 scores ≥3 and scores <3. Continuous variables were presented as mean ± standard deviation (SD) and compared using the independent sample t-test, whereas categorical variables were expressed as frequency and compared using the Pearson chi-square (χ2) test or Cochran-Mantel-Haenszel (CMH) chi-square (χ2) test or Fischer’s exact test. A stepwise selection technique in the binary logistic regression model was used for multivariate analysis to select factors associated with NRS 2002.
A scoring system was then developed from the 11 predictors in the model to weight each factor according to its effect size in association with NRS 2002 score ≥3. Points were assigned based on the relative strength of each factor: the smallest coefficient (0.375) was assigned 1 point, and each other risk factor was assigned a score by dividing its β by 0.375, rounded to the nearest integer. The resulting risk score was the sum of points assigned to the 11 individual predictors for a possible score ranging from 0 to 77. The discriminatory capability of the model was assessed by receiver operating characteristic (ROC) curve analysis. The NRS 2002 was used as the golden standard. All probability values were 2-tailed, and P values of 0.05 or less were considered statistically significant. The software SAS 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Clinical characteristics of included patients
During the study period, 15,460 patients diagnosed with PTBs were investigated, and 519 cases were excluded due to a lack of important information or integrity. A total of 14,941 cases were included in the final analysis. The flow diagram of patient inclusion is shown in Figure 1. Among 14,941 cases, 9,858 were male (66.00%), the overall average age was 47.49±18.22 years, BMI was 20.56±3.22 kg/m2, 1,866 cases (12.49%), had a history of drinking, 3,490 cases (23.36%) had the history of smoking, and 3,216 cases (21.52%) were complicated by extra-PTB. The other clinical characteristics and their proportion in detail concerning the site of extra-PTB, the complications, the lesions severity degree, the previous treatment history, and MTB etiological test results are shown in Table 1.
Table 1
| Characteristics | Included patients (n=14,941), n (%) |
|---|---|
| Sex (male) | 9,858 (66.00) |
| Age, years | 47.49±18.22 |
| BMI, kg/m2 | 20.56±3.22 |
| The history of drinking | 1,866 (12.49) |
| The history of smoking | 3,490 (23.36) |
| Complicated by extra-pulmonary TB | 3,216 (21.52) |
| Lymph node | 498 (3.33) |
| Intestines | 92 (0.62) |
| Tuberculous meningitis | 189 (1.26) |
| Tuberculous peritonitis | 147 (0.98) |
| Pleural tuberculosis | 1,104 (7.39) |
| Bronchial tuberculosis | 1,329 (8.89) |
| Newly treated cases1 | 11,242 (75.41) |
| Retreated cases1 | 2,808 (18.84) |
| Drug-resistant TB | |
| MDR-TB1 | 902 (6.05) |
| XDR-TB1 | 87 (0.58) |
| PDR-TB1 | 135 (0.91) |
| Comorbidities | |
| DM1 | 2,008 (13.47) |
| Hepatitis1 | 537 (3.60) |
| Drug-induced liver injury1 | 569 (3.82) |
| Liver cirrhosis1 | 100 (0.67) |
| Anemia1 | 59 (0.40) |
| Tumor | 180 (1.21) |
| Bacteriological feature | |
| AFB positive1 | 2,976 (19.96) |
| Culture positive1 | 4,762 (31.93) |
| PCR positive1 | 4,182 (28.05) |
| HIV positive1 | 45 (0.30) |
| Severity degree in lung fields | |
| ≥3 lung fields of lesions1 | 4,361 (29.25) |
| Cavity1 | 3,456 (23.18) |
| Diameter of cavity (≥3)2 | 518 (14.98) |
| Number of cavities ≥21 | 1,808 (12.13) |
The age and BMI are presented as mean ± SD. 1, means 33–34 cases having loss data in this item; 2, means 3,457 cases having loss data in this item. PTB, pulmonary tuberculosis; BMI, body mass index; TB, tuberculosis; MDR, multi-drug resistance; XDR, extent drug resistance; PDR, poly-drug resistance; DM, diabetes mellitus; AFB, acid-fast bacillus; PCR, polymerase chain reaction; HIV, human immunodeficiency virus; SD, standard deviation.
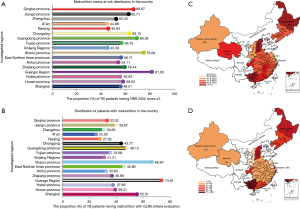
The distribution of PTB patients with nutritional risk in different areas of China
All 14,941 cases completed NRS 2002 and GLIM screening and evaluation. The criteria for both methods are shown in Table S1. The investigation and evaluation results can reflect the nutrition risk distribution of patients with active PTB in different regions of China. The results showed that the nutritional risk rate in patients with active PTB was 55.86% (8,346/14,941) by NRS 2002 and 40.27% by GLIM. Different regions had varied nutritional risk rates throughout the country; the highest nutritional risk by NRS 2002 and GLIM criteria was 81.63% and 73.81%, respectively, in the Guangxi province in southern China, the lowest was 44.88% and 25.20%, respectively, in Xi’an city, Shaanxi province of middle-western China, the regions with lower nutritional risk rate were Shanghai city, Nanjing city, and Xinjiang autonomous region, with 48.21%, 45.94%, and 45.39%, respectively; the regions with comparatively higher nutritional risk rates were the Shaanxi province at 75%, Qinghai province at 66.67%, and Chongqing city at 63.15%. The details, including NRS 2002 and GILM results in all regions are shown in Figure 2A-2D.
Univariate analysis of clinical factors closed associated with malnutrition in PTB
Univariate analysis was conducted to screen the clinical factors closely associated with nutritional risk. Based on the NRS 2002 tests, the results showed that 32 clinical factors, including patients with older age, low BMI, drinking, smoking, coexisting intestinal TB or brain TB, peritoneal TB, pleural TB, skin TB, coexisting extra-PTB (≥2 sites), retreated PTB, smear positivity, culture and molecular test positivity, PDR-TB, MDR-TB, RR-TB (except MDR or XDR-TB), coexisted DM, HIV infection, taking immunosuppressive agents, coexisting other pulmonary diseases, severe pneumonia, weight loss, decreased food intake, decreased blood lymphocyte count, inaccurate BMI due to severe edema, CD4/CD8 <1, lesions ≥50% lung fields, cavity and several cavities ≥3, high triglycerides, and total cholesterol had a significant association with having nutritional risk (P<0.05). The detailed data is shown in Table 2.
Table 2
| Factors | NRS 2002 <3 score, n (%) | NRS 2002 ≥3 score, n (%) | Statistical method | Statistical value | P value |
|---|---|---|---|---|---|
| Sex | Chi-square | 2.00 | 0.1575 | ||
| Male | 4,392 (66.60) | 5,466 (65.49) | |||
| Female | 2,203 (33.40) | 2,880 (34.51) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Age, years | CMH chi-square | 16.17 | <0.0001* | ||
| <18 | 2 (0.03) | 3 (0.04) | |||
| [18–40) | 2,359 (35.77) | 3,387 (40.59) | |||
| [40–60) | 2,619 (39.72) | 2,238 (26.82) | |||
| [60–80) | 1,482 (22.47) | 2,335 (27.98) | |||
| ≥80 | 132 (2.00) | 382 (4.58) | |||
| Total | 6,594 (100.00) | 8,345 (100.00) | |||
| BMI, kg/m2 | CMH chi-square | 9,584.01 | <0.0001* | ||
| >20.5 | 6,444 (98.10) | 753 (9.03) | |||
| 18.5–20.5 | 76 (1.16) | 3,668 (43.98) | |||
| <18.5 | 49 (0.75) | 3,919 (46.99) | |||
| Total | 6,569 (100.00) | 8,340 (100.00) | |||
| Drink | Chi-square | 9.75 | 0.0018* | ||
| Yes | 761 (11.54) | 1,105 (13.24) | |||
| No | 5,834 (88.46) | 7,241 (86.76) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Smoke | Chi-square | 18.85 | <0.0001* | ||
| Yes | 1,429 (21.67) | 2,061 (24.69) | |||
| No | 5,166 (78.33) | 6,285 (75.31) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Extra-pulmonary TB | Chi-square | 0.30 | 0.5870 | ||
| Yes | 1,406 (21.32) | 1,810 (21.69) | |||
| No | 5,189 (78.68) | 6,536 (78.31) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Intestinal TB | Chi-square | 22.67 | <0.0001* | ||
| Yes | 18 (0.27) | 74 (0.89) | |||
| No | 6,577 (99.73) | 8,272 (99.11) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Brain TB | Chi-square | 9.62 | 0.0019* | ||
| Yes | 62 (0.94) | 126 (1.51) | |||
| No | 6,533 (99.06) | 8,220 (98.49) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Peritoneal TB | Chi-square | 12.08 | 0.0005* | ||
| Yes | 40 (0.61) | 96 (1.15) | |||
| No | 6,555 (99.39) | 8,250 (98.85) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Lymph node TB | Chi-square | 2.02 | 0.1552 | ||
| Yes | 228 (3.46) | 254 (3.04) | |||
| No | 6,367 (96.54) | 8,092 (96.96) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Bone TB | Chi-square | 0.85 | 0.3555 | ||
| Yes | 6 (0.09) | 12 (0.14) | |||
| No | 6,589 (99.91) | 8,334 (99.86) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Pleural TB | Chi-square | 7.29 | 0.0069* | ||
| Yes | 433 (6.57) | 644 (7.72) | |||
| No | 6,162 (93.43) | 7,702 (92.28) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Skin TB | Chi-square | 12.24 | 0.0005* | ||
| Yes | 12 (0.18) | 1 (0.01) | |||
| No | 6,583 (99.82) | 8,345 (99.99) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Urinary TB | Chi-square | 0.00 | 0.9755 | ||
| Yes | 18 (0.27) | 23 (0.28) | |||
| No | 6,577 (99.73) | 8,323 (99.72) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Extra-pulmonary TB (≥2 cites) | Chi-square | 10.26 | 0.0014* | ||
| Yes | 148 (2.24) | 259 (3.10) | |||
| No | 6,447 (97.76) | 8,087 (96.90) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Newly or retreated TB | Chi-square | 44.26 | <0.0001* | ||
| Newly | 5,411 (83.27) | 6,447 (78.92) | |||
| Retreated | 1,087 (16.73) | 1,722 (21.08) | |||
| Total | 6,498 (100.00) | 8,169 (100.00) | |||
| Smear | Chi-square | 111.69 | <0.0001* | ||
| Negative | 5,187 (78.65) | 5,930 (71.05) | |||
| Positive | 1,408 (21.35) | 2,416 (28.95) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Culture | Chi-square | 121.08 | <0.0001* | ||
| Negative | 4,440 (67.32) | 4,886 (58.54) | |||
| Positive | 2,155 (32.68) | 3,460 (41.46) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Molecular tests | Chi-square | 119.47 | <0.0001* | ||
| Negative | 1,883 (47.93) | 1,791 (36.40) | |||
| Positive | 2,046 (52.07) | 3,129 (63.60) | |||
| Total | 3,929 (100.00) | 4,920 (100.00) | |||
| PDR (except RFP resistance) | Chi-square | 14.44 | 0.0001* | ||
| No | 6,459 (97.95) | 8,089 (96.96) | |||
| Yes | 135 (2.05) | 254 (3.04) | |||
| Total | 6,594 (100.00) | 8,343 (100.00) | |||
| RFP-resistance (except MDR/XDR-TB/PDR-TB) | Chi-square | 8.33 | 0.0039* | ||
| No | 6,467 (98.06) | 8,124 (97.34) | |||
| Yes | 128 (1.94) | 222 (2.66) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| MDR-TB | Chi-square | 16.18 | <0.0001* | ||
| No | 6,227 (94.42) | 7,744 (92.79) | |||
| Yes | 368 (5.58) | 602 (7.21) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| PDR-TB | Chi-square | 1.49 | 0.2226 | ||
| No | 6,542 (99.20) | 8,263 (99.01) | |||
| Yes | 53 (0.80) | 83 (0.99) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| XDR-TB | Chi-square | 1.28 | 0.2578 | ||
| No | 6,561 (99.48) | 8,291 (99.34) | |||
| Yes | 34 (0.52) | 55 (0.66) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| DM | Chi-square | 87.79 | <0.0001* | ||
| Yes | 1,075 (16.30) | 922 (11.05) | |||
| No | 5,520 (83.70) | 7,424 (88.95) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Chronic hepatitis | Chi-square | 0.48 | 0.4865 | ||
| Yes | 227 (3.44) | 305 (3.65) | |||
| No | 6,368 (96.56) | 8,041 (96.35) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Liver cirrhosis | Chi-square | 0.88 | 0.3469 | ||
| Yes | 20 (0.30) | 33 (0.40) | |||
| No | 6,575 (99.70) | 8,313 (99.60) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Transplantation | Chi-square (corrected) | 0.15 | 0.6954 | ||
| Yes | 6 (0.09) | 5 (0.06) | |||
| No | 6,589 (99.91) | 8,341 (99.94) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| HIV infection | Chi-square | 10.67 | 0.0011* | ||
| Yes | 9 (0.14) | 36 (0.43) | |||
| No | 6,586 (99.86) | 8,310 (99.57) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Abdominal surgery | Chi-square | 1.75 | 0.1853 | ||
| Yes | 21 (0.32) | 38 (0.46) | |||
| No | 6,574 (99.68) | 8,308 (99.54) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Immunosuppressive agents | Chi-square | 10.01 | 0.0016* | ||
| Yes | 14 (0.21) | 45 (0.54) | |||
| No | 6,581 (99.79) | 8,301 (99.46) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Other chronic pulmonary diseases | Chi-square | 84.35 | <0.0001* | ||
| Yes | 252 (3.82) | 614 (7.36) | |||
| No | 6,343 (96.18) | 7,732 (92.64) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Dialysis | Chi-square | 1.50 | 0.2211 | ||
| Yes | 8 (0.12) | 17 (0.20) | |||
| No | 6,587 (99.88) | 8,329 (99.80) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Malignant disease | Chi-square | 0.72 | 0.3977 | ||
| Yes | 73 (1.11) | 105 (1.26) | |||
| No | 6,522 (98.89) | 8,241 (98.74) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Hematological malignance | Fischer’s exact test | – | 1.0000 | ||
| Yes | 0 (0.00) | 0 (0.00) | |||
| No | 6,595 (100.00) | 8,346 (100.00) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Severe pneumonia | chi-square | 26.55 | <0.0001* | ||
| Yes | 29 (0.44) | 103 (1.23) | |||
| No | 6,566 (99.56) | 8,243 (98.77) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Stroke | chi-square | 0.07 | 0.7955 | ||
| Yes | 52 (0.79) | 69 (0.83) | |||
| No | 6,543 (99.21) | 8,277 (99.17) | |||
| Total | 6,595 (100.00) | 8,346 (100.00) | |||
| Weight loss | CMH chi-square | 680.46 | <0.0001* | ||
| No obvious change | 5,703 (91.39) | 5,427 (68.39) | |||
| Reduced >5% in one month | 16 (0.26) | 713 (8.99) | |||
| Reduced >5% in two months | 1 (0.02) | 381 (4.80) | |||
| Reduced >5% in three months | 520 (8.33) | 1,414 (17.82) | |||
| Total | 6,240 (100.00) | 7,935 (100.00) | |||
| Food intake in one week | CMH chi-square | 1,040.72 | <0.0001* | ||
| No obvious change | 5,767 (92.42) | 5,606 (70.66) | |||
| Decreased 25–50% | 471 (7.55) | 1,945 (24.51) | |||
| Decreased 51–75% | 2 (0.03) | 294 (3.71) | |||
| Decreased 76–100% | 0 (0.00) | 89 (1.12) | |||
| Total | 6,240 (100.00) | 7934 (100.00) | |||
| No accurate BMI due to severe edema | Chi-square | 6.15 | 0.0131* | ||
| Yes | 84 (1.35) | 149 (1.88) | |||
| No | 6,154 (98.65) | 7,773 (98.12) | |||
| Total | 6,238 (100.00) | 7,922 (100.00) | |||
| Lymphocyte count(absolute) | CMH chi-square | 395.76 | <0.0001* | ||
| >4 | 24 (0.38) | 32 (0.40) | |||
| 1–4 | 5,249 (83.19) | 5,479 (68.49) | |||
| <1 | 1,037 (16.43) | 2,489 (31.11) | |||
| Total | 6,310 (100.00) | 8,000 (100.00) | |||
| CD4/CD8 <1 | Chi-square | 4.13 | 0.0422* | ||
| Yes | 1,618 (86.85) | 1,806 (84.59) | |||
| No | 0 (0.00) | 0 (0.00) | |||
| Total | 1,863 (100.00) | 2,135 (100.00) | |||
| IGRAs | Chi-square | 1.17 | 0.2794 | ||
| Positive | 3,506 (81.57) | 4,084 (82.44) | |||
| Negative | 792 (18.43) | 870 (17.56) | |||
| Total | 4,298 (100.00) | 4,954 (100.00) | |||
| Lesions ≥50% lung fields | Chi-square | 83.80 | <0.0001* | ||
| Yes | 1,518 (24.70) | 2,462 (31.77) | |||
| No | 4,628 (75.30) | 5,288 (68.23) | |||
| Total | 6,146 (100.00) | 7,750 (100.00) | |||
| Cavity | Chi-square | 82.57 | <0.0001* | ||
| Has | 1,292 (20.72) | 2,164 (27.32) | |||
| No | 4,945 (79.28) | 5,756 (72.68) | |||
| Total | 6,237 (100.00) | 7,920 (100.00) | |||
| Any one cavity ≥3 cm in diameter | Chi-square | 2.73 | 0.0984 | ||
| Yes | 195 (13.77) | 323 (15.81) | |||
| No | 1,221 (86.23) | 1,720 (84.19) | |||
| Total | 1,416 (100.00) | 2,043 (100.00) | |||
| Number of cavities ≥3 | Chi-square | 80.66 | <0.0001* | ||
| Yes | 377 (6.86) | 811 (11.62) | |||
| No | 5,115 (93.14) | 6,168 (88.38) | |||
| Total | 5,492 (100.00) | 6,979 (100.00) | |||
| Triglyceride | Rank-Sam | Z=14.65 | <0.0001* | ||
| N (missing) | 1,885 (5E3) | 2,657 (6E3) | |||
| Mean (SD) | 1.54 (4.76) | 2.09 (34.48) | |||
| Median | 1.11 | 0.91 | |||
| Q1, Q3 | 0.82, 1.56 | 0.70, 1.22 | |||
| Min, max | 0.19, 159.00 | 0.07, 1,634.00 | |||
| Total cholesterol | Rank-Sam | Z=10.92 | <0.0001* | ||
| N (missing) | 1,891 (5E3) | 2,668 (6E3) | |||
| Mean (SD) | 6.39 (90.00) | 7.16 (107.90) | |||
| Median | 4.18 | 3.85 | |||
| Q1, Q3 | 3.60, 4.81 | 3.26, 4.51 | |||
| Min, max | 0.69, 3,917.00 | 0.49, 4,643.00 | |||
*, P<0.05. PTB, pulmonary tuberculosis; NRS 2002, Nutrition Risk Screening 2002; BMI, body mass index; TB, tuberculosis; PDR, poly-drug resistance; RFP, rifampicin; MDR, multi-drug resistance; XDR, extent drug resistance; DM, diabetes mellitus; HIV, human immunodeficiency virus; IGRAs, interferon gamma release assays; SD, standard deviation.
Multivariable analysis of clinical factors being independent risk factors of PTB patients having nutritional risk
The binary logistic regression model was implemented for multivariable analysis based on univariate analysis results; the data showed that 11 factors, including advanced age, decreased BMI, coexisting DM or severe pneumonia or HIV, decreased food intake within a week, absolute lymphocyte count decreased, taking immunosuppressive agents, weight loss, dialysis, and coexisting pleural TB were the independent influencing factors of malnutrition for patients with active PTB. Coexisting DM had the highest odds ratio (OR) value of 0.767, followed by weight loss (0.709), pleural TB (0.687), age (0.622), and absolute lymphocyte count (0.509) in turn; the lowest OR value was 0.002 in BMI. More detailed data are shown in Table 3.
Table 3
| Variable | Regression coefficient | Standard errors | Wald statistics | P values | OR (95% CI) |
|---|---|---|---|---|---|
| Constant term | −21.064 | 1.135 | 344.432 | <0.001 | |
| Age | 0.475 | 0.050 | 89.090 | <0.001 | 0.622 (0.564, 0.687) |
| BMI | 6.047 | 0.111 | 2,975.462 | <0.001 | 0.002 (0.002, 0.003) |
| DM | 0.265 | 0.100 | 7.059 | 0.008 | 0.767 (0.630, 0.933) |
| Severe pneumonia | 1.091 | 0.397 | 7.565 | 0.006 | 0.336 (0.154, 0.731) |
| HIV | 1.870 | 0.584 | 10.260 | 0.001 | 0.154 (0.049, 0.484) |
| Food intake within a week | 1.809 | 0.088 | 419.768 | <0.001 | 0.164 (0.138, 0.195) |
| Lymphocyte absolute count | 0.675 | 0.094 | 51.864 | <0.001 | 0.509 (0.424, 0.612) |
| Taking immunosuppressive agents | 1.632 | 0.487 | 11.247 | <0.001 | 0.195 (0.075, 0.507) |
| Loss weight | 0.343 | 0.038 | 81.671 | <0.001 | 0.709 (0.658, 0.764) |
| dialysis | 1.760 | 0.599 | 8.626 | 0.003 | 0.172 (0.053, 0.557) |
| Pleural TB | 0.375 | 0.141 | 7.093 | 0.008 | 0.687 (0.521, 0.906) |
PTB, pulmonary tuberculosis; OR, odds ratio; CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; HIV, human immunodeficiency virus; TB, tuberculosis.
Construction of a new screening model of nutritional risk for patients with active PTB based on NRS 2002 standard.
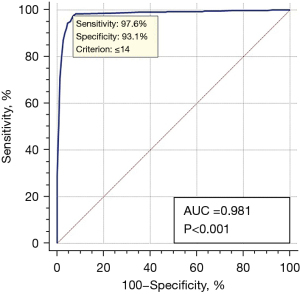
Since we implemented the screening based on the NRS 2002 score standard, we identified 14 factors listed in the NRS 2002 score list that were not in the list of factors with statistical significance in the present results. Therefore, we hypothesized that a more appropriate screening model, especially for active PTB, should be built from the analysis of the present large samples we had investigated. Based on factors screened by multivariable factors analysis, parameters for each factor were calculated, as shown in Table 4. The scores added up from all parameters were the final scores for each evaluation of nutritional risk; the maximum score was 77 if patients had been assessed as having the maximum level of these 11 factors; the minimum score was 0 if patients had not been assessed as having the minimum level in these 11 factors. Using the NRS 2002 screening test as a gold standard and score 14 as the cut-off value of having nutritional risk or not having nutritional risk, the sensitivity and specificity of the new model for the diagnosis of PTB patients having nutritional risk were 97.6% and 93.1%, respectively, and the area under the curve (AUC) was 0.981, P<0.001. These findings demonstrated that the new model could be appropriately used to screen the nutrition risk for a patient with active PTB as it included many factors designed explicitly for active TB rather than general hospitalized patients screened by the NRS 2002 list (Figure 3).
Table 4
| Variable | Categories | Reference value | β | P | Score |
|---|---|---|---|---|---|
| Age (years) | 0.475 | ||||
| <18 | 1 | 0 | 0 | ||
| [18–40) | 2 | 0.475 | 1 | ||
| [40–60) | 3 | 0.95 | 3 | ||
| [60–80) | 4 | 1.425 | 4 | ||
| ≥80 | 5 | 1.9 | 5 | ||
| BMI | 6.047 | ||||
| >20.5 | 1 | 0 | 0 | ||
| 18.5–20.5 | 2 | 6.047 | 16 | ||
| <18.5 | 3 | 12.094 | 32 | ||
| DM | 0.265 | ||||
| No | 0 | 0 | 0 | ||
| Yes | 1 | 0.265 | 1 | ||
| Severe pneumonia | 1.091 | ||||
| No | 0 | 0 | 0 | ||
| Yes | 1 | 1.091 | 3 | ||
| HIV | 1.870 | 0 | |||
| No | 0 | 0 | 0 | ||
| Yes | 1 | 1.870 | 5 | ||
| Food intake within a week | 1.809 | ||||
| No obvious change | 1 | 0 | 0 | ||
| Decreased 25–50% | 2 | 1.809 | 5 | ||
| Decreased 51–75% | 3 | 3.618 | 10 | ||
| Decreased 76–100% | 4 | 5.427 | 14 | ||
| Lymphocyte absolute count (level) | 0.675 | ||||
| >4 | 1 | 0 | 0 | ||
| 1–4 | 2 | 0.675 | 2 | ||
| <1 | 3 | 1.35 | 4 | ||
| Taking immunosuppressive agents | 1.632 | ||||
| No | 0 | 0 | 0 | ||
| Yes | 1 | 1.632 | 4 | ||
| Weight loss | 0.343 | ||||
| No obvious change | 1 | 0 | 0 | ||
| Decreased >5% in 1 month | 2 | 0.343 | 1 | ||
| Decreased >5% in 2 months | 3 | 0.686 | 2 | ||
| Decreased >5% in 3 months | 4 | 1.029 | 3 | ||
| Dialysis | 1.760 | ||||
| No | 0 | 0 | 0 | ||
| Yes | 1 | 1.760 | 5 | ||
| Pleural TB | 0.375 | ||||
| No | 0 | 0 | 0 | ||
| Yes | 1 | 0.375 | 1 | ||
NRS 2002, Nutrition Risk Screening 2002; BMI, body mass index; DM, diabetes mellitus; HIV, human immunodeficiency virus; TB, tuberculosis.
The comparison of GLIM criteria with NRS 2002 tests
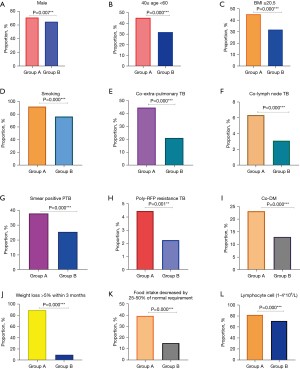
Apart from 32 cases lacking information for evaluation by GLIM, 14,909 cases were effectively evaluated by GLIM criteria. According to GLIM, the positive rate of malnutrition was 42.7% (6,016/14,090); 583 cases were deemed not having nutritional risk when screened by NRS 2002 but were positively screened by GLIM, whereas 2,907 cases were positively screened by NRS 2002 yet negatively screened by GLIM; 24.77% (3,490/14,090) of the cases were inconsistent with NRS 2002 and GLIM standards. The agreement of NRS 2002 and GLIM for screening the nutritional risk among the patients with active PTB was moderate (κ value 0.54); the results indicated that 3.91% (583/14,909) of patients have the possibility of being missed by screening with NRS 2002. After comparison and analysis, we found that 12 clinical factors (male, aged 40–60 years, smoking, BMI ≥20.5 kg/m2, coexisting extra-PTB, coexisting lymph node TB, DM, smear positive, RFP resistance, weight loss >5% within three months, food intake decreased by 25–50%, and lymphocyte count at [1–4]×109/L were significantly higher in NRS 2002−/GLIM+ cases than those in the remaining cases (P<0.05), implying that patients having any 1 of these 12 clinical factors should be screened by both methods at the same time and those not having 12 clinical factors can be easily negatively screened by NRS 2002 and positively evaluated by GLIM. The details are displayed in Figure 4A-4L.
Discussion
PTB is a chronic infectious and consumptive disease; study has pointed out that malnutrition is widespread among TB patients as they are likely to have malabsorption, which can directly influence the energy intake that results in proteolysis and lipolysis; the latter is closely related to immune regulation in the host (19). However, how to improve or correct the malnutrition status of active TB patients has remained unclear in many hospitals and physicians. Although screening the malnutrition patients first is essential, few studies have been conducted to screen patients with malnutrition or nutritional risk among active TB patients. The GLIM and NRS 2002 screening methods have been the most commonly used to evaluate the nutritional status of the general population; however, which method should be better for diagnosing patients with nutritional risk among those with active TB also remain unknown; meanwhile, there are appropriate means of identification of malnutrition or nutritional risk methods for other diseases, such as the malnutrition screening tool (MST), or NRS for cancer treatment (20), MST, Malnutrition Universal Screening Tool (MUST), or Short Nutritional Assessment Questionnaire (SNAQ) in screening nutritional risk for patients in the emergency department (21), and the Controlling Nutritional Status (CONUT) Index for digestive diseases (22). Therefore, the second aim of the present study was to compare and choose which method is better to screen the malnutrition or nutritional risk for active TB. Because TB patients had their specific clinical characteristics, besides general screening factors, the more appropriate evaluation model specifically for TB patients was another aim of the present study.
The data on nutritional risk distribution in the whole country implied an imbalance of nutritional risk or malnutrition among different cities in different provinces. The highest nutritional risk was as high as 81.63% in Nanning city of Guangxi province, the lowest nutritional risk was 44.88% in Xi’an of Shaanxi province, and the average nutritional risk rate of active PTB was 55.86% in China, demonstrating that there is severe malnutrition status in patients with active TB. The underlying reasons for imbalanced nutritional status among TB patients were complicated, associated with different medical attention to nutritional supplement, economical level, TB management, etc. Considerable attention should be given to these types of patient populations.
The data from univariate analysis based on NRS 2002 screening showed that besides seven factors, including age, BMI, diabetes, weight loss, food intake, severe pneumonia, and other chronic pulmonary diseases which were included in the screening list of NRS 2002 standard, the additional 25 clinical factors were statistically higher in patients with NRS 2002 score ≥3 than those in patients with NRS 2002 <3 score, which included drinking, smoking, intestinal TB, brain TB, peritoneal TB, pleural TB, skin TB, extra-PTB (≥two sites), retreated PTB, smear or culture or molecular positive, PDR-TB, MDR-TB, HIV infection, severe edema, taking immunosuppressive agents, absolute lymphocyte count decrease, CD4/CD8 <1, lesions ≥50% lung fields, the presence of a cavity, number of cavities ≥3, higher total cholesterol, or triglyceride. These factors were indeed closely associated with the severity of tuberculous diseases or had conversely association with treatment outcomes in active TB; for example, a pulmonary cavity or a higher number of the cavity in the lungs often implies increased transmission, unfavorable treatment outcomes, and increased bacillus burden in the local lesions, or impaired cell-mediated protective immunity of host against MTB (23-27). TB is a disease that mainly invades the lungs but often disseminates to extra-PTB, including almost every site of the body; the most common extra-pulmonary sites are lymph nodes, bone, brain, pleura, peritoneal cavity, or skin. This univariate analysis showed that five sites of tuberculous lesions were involved, including brain or meningeal, intestinal, pleural, and peritoneal. Skin TB was significantly associated with nutritional risk. In contrast, other sites of TB, such as lymph nodes or the urinary system, had not been significantly associated with nutritional risk, implying that tuberculous lesions in these organs are more likely to pose an obstacle to or influence nutrition absorption at any disease stage, such as in meningitis (28). More significant than three organs of extra-pulmonary invasion also imply the severity of TB lesions in patients. Other clinical characteristics such as drug-resistant TB and decreased lymphocyte count were found to be significantly higher in patients with NRS 2002 score ≥3 than those with a score <3; these factors are specifically unique for TB patients, and the significant differences indicated that these factors might be used in the evaluation or diagnosis of nutritional risk for patients with active PTB.
The results from the multivariate analysis indicated that 21 factors with statistically significant differences in the univariate analysis did not enter into the multivariate results. Finally, 11 factors became the independent risk factors of nutritional risk in PTB (age, BMI, DM, lymphocyte counts, and so on). Bacteriological tests failed to enter the multivariate model analysis because there were too low favorable rates of AFB, culture, and molecular tests due to the limited quality of sputum samples or laboratory conditions, which might influence the positive rate of the tests (29-31). The exact reasons applied to the lack of the factor of drug-resistance TB.
To determine whether these risk factors identified by multivariate analysis can be applied in screening or diagnostic testing for nutritional risk for active PTB patients, we further established the diagnostic model by calculating the score based on the statistical value, as shown in Table 4. Each factor has several scores according to the reference value. We calculated the diagnostic value and determined that the sensitivity and specificity of the new model were as high as 97.6% and 93.1%, respectively, using score 14 as cut off value, which implied that this diagnostic model has a value similar to that of the NRS 2002 for screening nutritional risk. In contrast, NRS 2002 screening does not incorporate characteristics specifically for TB patients, such as pleural TB. In addition, some factors lacking in the NRS 2002 list, such as taking immunosuppressive agents and decreased lymphocyte count, were important in the new model for TB patients and calculated by the present study.
A comparison of the GLIM and NRS 2002 to screen the active PTB patients indicated that NRS 2002 is superior for diagnosing patients with nutritional risk and that the new model we constructed is also more appropriate to identify patients with nutrition risk as the total score can be 74, a physician might judge the nutritional risk according to the degree of the scores. GLIM can be seen as a suitable method for the diagnosis of malnutrition in all patients, such as those with Crohn’s disease and cardiovascular diseases (32,33). Study has also focused on comparing the value of GLIM and NRS 2002 and reported GLIM is acceptable for malnutrition diagnosis, whereas NRS 2002 is appropriate to screen out patients with nutritional risk (34); however, in the present study, the incomplete agreement indicated that further study should be carried out to decide how to achieve the best screening efficacy for active TB patients with nutritional risk. The previously most favorable view had pointed out that the first step of the nutritional screening process should be the NRS 2002, followed by GLIM or subjective global assessment (SGA) for the diagnosis of malnutrition; however, it should ideally be on the premise of no false negative diagnosis occurring during the screening step. Our data showed that there were 583 (3.9%) cases with NRS 2002 negative screening results but with positive GLIM criteria; the explanation might be that TB belongs to the category of chronic disease, which scores 1 in the etiology in the GLIM criteria, implying that TB patients had a greater chance of having positive evaluation results by GLIM. This 3.9% of patients were likely to be missed by NRS 2002 and should be screened by both methods in case of being missed. Our further analysis results yielded the valid suggestion that patients having any 1 of above 12 factors should be screened by both methods in case of being missed, and the remaining patients can be firstly screened by NRS 2002 for nutritional risk and then evaluated by GLIM for malnutrition.
The present study had several limitations. It was a retrospective study, and the assessment of factors such as change in food intake within several months might be inaccurate as physicians only obtained the information from the medical records. Additionally, the cases for the present study in different hospitals were not even; many cases were concentrated in eastern and central China, whereas northern China had fewer cases which might influence the results finally calculated.
Conclusions
In conclusion, the malnutrition status of active PTB patients is severe in China. Eleven clinical factors should be screened for nutritional risk and malnutrition evaluation as early as possible. We recommend the model we constructed to screen for nutritional risk among TB patients as it is closer to TB clinical characteristics than NRS 2002.
Acknowledgments
We thank all participants for their time and efforts.
Funding: This study was supported by the National Natural Science Foundation of China (No. 82170006), the Shanghai Science and Technology Committee Fund (Nos. 21Y11901000 and 20ZR1446700), and the Clinical Research Foundation of Shanghai Pulmonary Hospital (Nos. FKLY20017 and SKPY2021003). The authors declare all the sources of funding, including financial support, in their manuscript. The authors confirm that the information in the above funding statement is accurate and was allocated in accordance with the funder’s requirement.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-623/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-623/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-623/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-623/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study conformed to the Declaration of Helsinki (as revised in 2013) for ethical principles for research. This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (No. K20-431), the ethics committee waived the requirement for written informed consent on the proviso that the privacy of patients enrolled was protected throughout the present study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Global Tuberculosis report. Geneva: WHO, 2021.
- World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organization; 2017 Licence: CC BY-NCSA 30 IGO; 2017.
- Marín Franco JL, Genoula M, Corral D, et al. Host-Derived Lipids from Tuberculous Pleurisy Impair Macrophage Microbicidal-Associated Metabolic Activity. Cell Rep 2020;33:108547. [Crossref] [PubMed]
- Braverman J, Sogi KM, Benjamin D, et al. HIF-1α Is an Essential Mediator of IFN-γ-Dependent Immunity to Mycobacterium tuberculosis. J Immunol 2016;197:1287-97. [Crossref] [PubMed]
- Shaji B, Arun Thomas ET, Sasidharan PK. Tuberculosis control in India: Refocus on nutrition. Indian J Tuberc 2019;66:26-9. [Crossref] [PubMed]
- Ortblad KF, Salomon JA, Bärnighausen T, et al. Stopping tuberculosis: a biosocial model for sustainable development. Lancet 2015;386:2354-62. [Crossref] [PubMed]
- Kant S, Gupta H, Ahluwalia S. Significance of nutrition in pulmonary tuberculosis. Crit Rev Food Sci Nutr 2015;55:955-63. [Crossref] [PubMed]
- Sinha P, Lakshminarayanan SL, Cintron C, et al. Nutritional Supplementation Would Be Cost-Effective for Reducing Tuberculosis Incidence and Mortality in India: The Ration Optimization to Impede Tuberculosis (ROTI-TB) Model. Clin Infect Dis 2022;75:577-85. [Crossref] [PubMed]
- Grobler L, Nagpal S, Sudarsanam TD, et al. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev 2016;2016:CD006086. [Crossref] [PubMed]
- Li Y, Yang F, Zhou H, et al. Clinical application of NRS-2002 in nutritional risk screening of tuberculosis inpatients. Ann Palliat Med 2021;10:5322-8. [Crossref] [PubMed]
- Budzyński J, Tojek K, Czerniak B, et al. Scores of nutritional risk and parameters of nutritional status assessment as predictors of in-hospital mortality and readmissions in the general hospital population. Clin Nutr 2016;35:1464-71. [Crossref] [PubMed]
- Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207-17. [Crossref] [PubMed]
- Cederholm T, Jensen GL. To create a consensus on malnutrition diagnostic criteria: A report from the Global Leadership Initiative on Malnutrition (GLIM) meeting at the ESPEN Congress 2016. Clin Nutr 2017;36:7-10. [Crossref] [PubMed]
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. [Crossref] [PubMed]
- Sanson G, Sadiraj M, Barbin I, et al. Prediction of early- and long-term mortality in adult patients acutely admitted to internal medicine: NRS-2002 and beyond. Clin Nutr 2020;39:1092-100. [Crossref] [PubMed]
- Jensen GL, Cederholm T, Correia MITD, et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. JPEN J Parenter Enteral Nutr 2019;43:32-40. [Crossref] [PubMed]
- World Health Organization. Treatment of Tuberculosis guidelines Fourth Edition. Geneva; 2010.
- 2016 WHO. WHO treatment guidelines for drug-resistant Tuberculosis 2016 updates. Geneva. 2016.
- Akkerman OW, Ter Beek L, Centis R, et al. Rehabilitation, optimized nutritional care, and boosting host internal milieu to improve long-term treatment outcomes in tuberculosis patients. Int J Infect Dis 2020;92S:S10-4. [Crossref] [PubMed]
- Castillo-Martínez L, Castro-Eguiluz D, Copca-Mendoza ET, et al. Nutritional Assessment Tools for the Identification of Malnutrition and Nutritional Risk Associated with Cancer Treatment. Rev Invest Clin 2018;70:121-5. [Crossref] [PubMed]
- Rabito EI, Marcadenti A, da Silva Fink J, et al. Nutritional Risk Screening 2002, Short Nutritional Assessment Questionnaire, Malnutrition Screening Tool, and Malnutrition Universal Screening Tool Are Good Predictors of Nutrition Risk in an Emergency Service. Nutr Clin Pract 2017;32:526-32. [Crossref] [PubMed]
- Chávez-Tostado M, Cervantes-Guevara G, López-Alvarado SE, et al. Comparison of nutritional screening tools to assess nutritional risk and predict clinical outcomes in Mexican patients with digestive diseases. BMC Gastroenterol 2020;20:79. [Crossref] [PubMed]
- Kim SH, Shin YM, Yoo JY, et al. Clinical Factors Associated with Cavitary Tuberculosis and Its Treatment Outcomes. J Pers Med 2021;11:1081. [Crossref] [PubMed]
- Fan L, Xiao H, Mai G, et al. Impaired M. tuberculosis Antigen-Specific IFN-γ Response without IL-17 Enhancement in Patients with Severe Cavitary Pulmonary Tuberculosis. PLoS One 2015;10:e0127087. [Crossref] [PubMed]
- Imperiale BR, García A, Minotti A, et al. Th22 response induced by Mycobacterium tuberculosis strains is closely related to severity of pulmonary lesions and bacillary load in patients with multi-drug-resistant tuberculosis. Clin Exp Immunol 2021;203:267-80. [Crossref] [PubMed]
- Fan L, Shen H, Huang H, et al. Impairment of Wnt/β-catenin signaling in blood cells of patients with severe cavitary pulmonary tuberculosis. PLoS One 2017;12:e0172549. [Crossref] [PubMed]
- Evans EE, Avaliani T, Gujabidze M, et al. Long term outcomes of patients with tuberculous meningitis: The impact of drug resistance. PLoS One 2022;17:e0270201. [Crossref] [PubMed]
- Meyer AJ, Atuheire C, Worodria W, et al. Sputum quality and diagnostic performance of GeneXpert MTB/RIF among smear-negative adults with presumed tuberculosis in Uganda. PLoS One 2017;12:e0180572. [Crossref] [PubMed]
- Acuña-Villaorduña C, Orikiriza P, Nyehangane D, et al. Effect of previous treatment and sputum quality on diagnostic accuracy of Xpert® MTB/RIF. Int J Tuberc Lung Dis 2017;21:389-97. [Crossref] [PubMed]
- Shuaib YA, Khalil EAG, Schaible UE, et al. Smear Microscopy for Diagnosis of Pulmonary Tuberculosis in Eastern Sudan. Tuberc Res Treat 2018;2018:8038137. [Crossref] [PubMed]
- Fiorindi C, Luceri C, Dragoni G, et al. GLIM Criteria for Malnutrition in Surgical IBD Patients: A Pilot Study. Nutrients 2020;12:2222. [Crossref] [PubMed]
- Kootaka Y, Kamiya K, Hamazaki N, et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin Nutr 2021;40:146-52. [Crossref] [PubMed]
- Nakyeyune R, Ruan X, Wang X, et al. Comparative analysis of malnutrition diagnosis methods in lung cancer patients using a Bayesian latent class model. Asia Pac J Clin Nutr 2022;31:181-90. [PubMed]
- Correia MITD. Nutrition Screening vs Nutrition Assessment: What's the Difference? Nutr Clin Pract 2018;33:62-72. [Crossref] [PubMed]
(English Language Editor: J. Jones)



