
TAK-242 protects against oxygen-glucose deprivation and reoxygenation-induced injury in brain microvascular endothelial cells and alters the expression pattern of lncRNAs
Highlight box
Key findings
• TAK-242 alleviated OGD/R-induced injury in BMECs by altering expression of lncRNAs.
What is known and what is new?
• Previous study showed that OGD/R activated TLR4 signaling and induced pyroptosis and inflammation in BMECs.
• TAK-242 changed the expression pattern of lncRNAs in OGD/R cells and suppressed OGD-R-mediated NF-κB and NLRP3/Caspase-1 pathway activation in BMECs.
What is the implication, and what should change now?
• TAK-242 regulated AABR07049961.1, AC127076.2, AABR07066020.1, and AABR07025303.1-encoded short peptides during OGD/R.
Introduction
Deep hypothermic circulatory arrest (DHCA) is a technique used during the surgical treatment of aneurysms of the thoracic aorta in adult patients, and complex congenital heart disease in neonates (1,2). It involves cooling the patient’s core body temperature and stopping blood circulation (3). The cessation of bold flow provides an optimal visual field during surgery, and deep hypothermia protects cerebral and other vital organs from ischemia (3). Although the hypothermic state limits cerebral damage by lowering the global cerebral metabolism and oxygen consumption, DHCA-induced hypoxic-ischemic insult is still the leading cause of neuronal injury after surgery involving DHCA (3). Ischemia and hypoxia results in the excessive and hyperactivity of neuronal cells, which increase the expression of genes that promote oxidation and the caspase family genes to induce the apoptosis, and the adenosine triphosphate deletion leads neuronal cells necrosis (4). Therefore, efforts to develop effective adjunctive neuroprotective therapies are necessary.
Brain microvascular endothelial cells (BMECs) are essential components of the cerebrovascular network and participate in maintaining the blood-brain barrier (BBB) and brain function (5). They are highly responsive and sensitive to oxygen deprivation (6). Cerebral ischemia causes BMECs degeneration and dysfunction, resulting in degradation of endothelial tight junction and increased BBB permeability and the injury of neuronal cells (7,8). Moreover, BMECs have been shown to release various vasoactive and proinflammatory/inflammatory factors in response to ischemia. These factors induce platelet and neutrophil activation and adhesion, and enhance leukocyte adhesion and transmigration into the brain, resulting in the exacerbation of ischemic damage (9). Additionally, protection the BMECs contributed to reduced neuronal apoptosis (10,11). The important roles of BMECs in brain ischemic injury highlight the potential of BMECs-targeted therapy as an adjunctive neuroprotective therapy during DHCA.
In our previous studies, oxygen-glucose deprivation and reoxygenation (OGD/R)-treated rat primary BMECs were used to mimic the BMECs exhibiting from hypoxia-ischemia after DHCA, and we found that OGD/R treatment stimulated Toll-like receptor 4 (TLR4) protein expression, caspase-1-mediated pyroptosis, and the release of pro-inflammatory factors (12,13). TLR4, belonging to the family of pattern recognition receptors, plays an important role in innate and inflammatory responses (14). It can recognize pathogen-associated molecular patterns and damage-associated molecular patterns, and activate the production of pro-inflammatory nuclear factor κB (NF-κB) and subsequent inflammatory cytokines (15). A previous study found that TLR4/NF-κB inflammatory pathway maybe the signal pathway for treating epilepsy (16). Accumulating studies suggest that TLR4 plays a key role in cell death and inflammation injury in the brain following ischemia-reperfusion, and suppression of TLR4 could remarkably improve cerebral ischemia injury in animal models (17,18). Additionally, TLR4 affected many other pathways, such as MyD88/mTOR pathway, TRIF/TBK1/IRF3 pathway, and MAPK pathway etc. Therefore, TLR4 play an important role in different diseases (19-21).
Long non-coding RNAs (lncRNAs) are defined as RNA transcripts >200 nt in length that are not translated into functional proteins (22). They participate in multiple biological processes and exhibit various regulatory functions through epigenetic modification, transcriptional, chromatin remodeling, and mRNA integrity, and post-translational modification to regulate the mRNA expression or protein expression, which expression changes affected the downstream signaling networks (22). LncRNAs play dual role in cancer, for example, lncRNA-PART1 inhibits the progression of glioma, but promotes the lung cancer development (23). Recently, TLR4 has been shown to regulate inflammation via lncRNAs (24). LincRNA-EPS has emerged as a transcriptional brake to restrain inflammation (25). Research has shown that TLR4 suppresses lincRNA-EPS expression, which increases the chromatin accessibility at the promoters of immune response genes (IRGs), enhances IRGs expression, and promotes inflammation (25). Another study showed that TLR4 regulates lncRNA-F630028O10Rik in the secondary phase of spinal cord injury (SCI) mice (24). The study showed that lncRNA-F630028O10Rik was induced via the TLR4/MyD88/STAT1 signal complex, and that lncRNA functioned as a competitive endogenous RNA (ceRNA) for the miR-1231-5p/Col1a1 axis and enhanced microglial pyroptosis after SCI by activating the PI3K/AKT pathway (24). Therefore, we hypothesized that some lncRNAs were associated with pyroptosis and inflammation in OGD/R BMECs, and that TLR4 participated in these lncRNAs expression.
MicroRNAs (miRNAs) are small non-coding RNAs of around 18–24 nt in length. They regulate gene expression post-transcriptionally by binding to complementary sequences in the target messenger RNAs (mRNAs) (26). For example, the miR-200a-3p expression was promoted in oxygen-glucose deprivation-induced apoptosis of SHSY5Y, and inhibited the expression of miR-200a-3p promoted its’ target gene corin expression to promote the cell proliferation in oxygen-glucose deprivation treated SHSY5Y (27). Several lncRNAs have been found to harbor miRNAs binding sites; these lncRNAs can function as ceRNA to sponge miRNAs and regulate gene expression by sequestering miRNA from their targets (26,28). Moreover, several lncRNAs have been confirmed to have small open reading frames <300 nt in length, and could code for short peptides with biological functions (29,30). Emerging evidence indicates that some lncRNAs may have dual functions, RNA, and peptides (29,31). Thus, we speculated that several lncRNAs involved in OGD/R injury may function as ceRNAs or encode peptides.
Ethyl(6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242) is a small-molecule inhibitor of TLR4 which selectively binds to TLR4 and interferes with interactions between TLR4 and its adaptor molecules, blocking the signaling transduction and downstream signals activation (32). In the preclinical animal models, TAK-242 play an effective drug for the ischaemia-reperfusion injury in myocardial and liver (33,34). And a previous study showed in patients with sepsis, the TAK-242 was tested in clinical trials; however, due to the cost-effectiveness, its development has been terminated (35). Another study indicated that TAK-242 maybe a novel drug for the treatment of IR when combined with a nanotechnology-based drug delivery system (33).
In this study, we evaluated the role of TLR4 signaling in OGD/R-induced injury in BMECs by shutting off the signaling with TAK-242, and profiled the expression patterns of lncRNAs and mRNAs in BMECs (control group), OGD/R BMECs (OGD/R group), and TAK-242-treated OGD/R BMECs (TAK-242+OGD/R group) by next-generation sequencing. Further, liquid chromatography-tandem mass spectrometry (LC-MS/MS) was employed to detect and characterize the lncRNA-encoded peptides. These results maybe provide a new theory basis for the treatment of DHCA. We present this article in accordance with the MDAR reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-360/rc).
Methods
Preparation and cultivation of primary rat BMECs
Rat primary BMECs were isolated and cultured as previously described (12,13). Briefly, BMECs were isolated from 6-week-old rats with a Percoll (Pharmacia, Uppsala, Sweden) gradient, and cultured in collagen type IV/fibronectin-coated 35-mm plastic dishes with Dulbecco’s modified Eagle’s medium (DMEM) low-glucose (Gibco, Grand Island, NY, USA) at 37 ℃ and 5% CO2.
The rats were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). All animal experimental procedures complied with local and international guidelines for the care and use of animals and were approved by the Animal Ethics Welfare Committee of First Affiliated Hospital of Gannan Medical University (approval No. GFAC-AEWC-121).
Cell treatment
BMECs were divided into 3 groups: the control, OGD/R BMECs, and TAK-242+OGD/R groups. In the control group, cells were cultured in a growth culture medium under normoxic conditions. In the OGD/R group, the cells were rinsed twice with phosphate-buffered saline (PBS), and then cultured with glucose-free DMEM in a hypoxia chamber for 4 hours. Thereafter, an equal volume of DMEM high-glucose (Gibco) containing 10% fetal bovine serum (FBS; Gibco) was added to the cells, and culturing continued for 12 hours under normoxic conditions. In the TAK-242 + OGD/R groups, the cells were exposed to glucose-free DMEM and hypoxia condition for 4 hours. Subsequently, an equal volume of DMEM high-glucose medium containing 10% FBS and TAK-242 was added to the wells, and the cell was further cultured for 12 hours under normoxic conditions. The TAK-242 working concentration was 30 µM, same as previous study (36).
Cell viability assay
The cells were seeded in triplicate, allowed to adhere overnight, and then subjected to OGD/R or TAK-242 + OGD/R treatment. To detect cell viability, Cell Counting Kit-8 (CCK-8) assays were performed using a CCK-8 Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay
To detect the inflammatory factors secreted by BMECs, the cell supernatants of each group were collected after OGD/R treatment. The concentrations of interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) were measured using the enzyme-linked immunosorbent assay (ELISA) kit (Cusabio, Wuhan, China) according to the manufacturer’s instructions. The assays were performed in triplicate.
Western blot assay
The proteins were extracted from the cells with radioimmunoprecipitation assay (RIPA) buffer (Beyotime Biotechnology), and the concentration was measured using a bicinchoninic acid (BCA) kit (Beyotime Biotechnology). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to separate proteins and the cells were then transferred to the polyvinylidene fluoride membranes (PVDF; Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% bovine serum albumin (BSA) for 1 hour and incubated with TLR-4 (1:500, Beyotime Biotechnology), NLRP3 (1:500, Beyotime Biotechnology), p-p65 (1:500, Beyotime Biotechnology), Caspase-1 (1:500, Beyotime Biotechnology), or GAPDH (1:500, Beyotime Biotechnology) primary antibodies at 4 ℃ overnight. Subsequently, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:500, Beyotime Biotechnology) at room temperature for 1 hour, and then the bounded secondary antibody was visualized using chemiluminescence (Beyotime Biotechnology).
High-throughput sequencing and bioinformatics analysis
Total RNA was isolated from cells using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. The RNA integrity was evaluated using denaturing agarose gel electrophoresis. Subsequently, the RNA samples were sent to Novogen company (Beijing, China) for library construction, and then subjected to RNA deep sequencing on HiSeq3000 (Illumina, San Diego, CA, USA). Fold change >2 and adjusted P value <0.05 were used as thresholds to select the differentially expressed genes. The ceRNA networks were plotted using Cytoscape software V.3.8.2 (https://cytoscape.org/). To explore the associated biological pathways of the differentially expressed genes, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was conducted using the online platform of Database for Annotation, Visualization and Integrated Discovery (DAVID).
Quantitative reverse transcription polymerase chain reaction
The lncRNAs expression in the cells was validated by quantitative reverse transcription polymerase chain reaction (qRT-PCR). The total RNA was extracted from cells and reverse-transcribed into cDNA with PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, Tokyo, Japan). The qRT-PCR was performed using ChamQTM SYBR Color qPCR Master Mix (Vazyme, Jiangsu, China) following the manufacturer’s protocols. GAPDH was used as an internal reference and relative expression levels of the lncRNAs were calculated using the 2−∆∆Ct method. The primer sequences are listed in Table 1.
Table 1
| Primer name | Sequence (5’-3’) |
|---|---|
| AABR07000411.1-F | ACTGGGCACAGATGAGAACG |
| AABR07000411.1-R | CTGGTTGCTGACAAAGCACC |
| AABR07006957.1-F | AAACCACACCGAGGCAAAGT |
| AABR07006957.1-R | GGGCTCAACTCTGAGCACTG |
| AABR07008256.1-F | ATAGAACAGTGGCCTGGTGC |
| AABR07008256.1-R | GTCTGTTAGGTAGCGGAGCC |
| AABR07069473.1-F | CGCCCAGTAGCCATAGAGTG |
| AABR07069473.1-R | TCCAGCCTAAATCCAAGCCG |
| AC130862.4-F | CGGAGTCCAGAGAATTGGGG |
| AC130862.4-R | CCTTCCCTGCTTCTCCGTTT |
| LOC102549726-F | ATGGACTCTGGGGGACAAGA |
| LOC102549726-R | CTTCCGGAGTCCACAGACG |
| GAPDH-F | GAGTCAACGGATTTGGTCGT |
| GAPDH-R | GACAAGCTTCCCGTTCTCAG |
LC-MS/MS
The total proteins from control, OGD/R, and TAK-242+OGD/R BMECs were subjected to trypsin digestion, and Fitgene Biotech Co., Ltd. (Guangzhou, China) for LC-MC/MS analysis. Briefly, the proteins were separated by SDS-PAGE. The target bands were harvested and subjected to in-gel trypsin digestion and the digested peptides were extracted using C18 Zip Tip (Millipore, Burlington, MA, USA) and dissolved in 0.1% formic acid and 2% acetonitrile. The resulting peptides were loaded on an Acclaim PepMap RSLC C18 column (300 µm × 5 mm, 5 µm, 100 Å, Thermo Fisher, Waltham, MA, USA, 160454) and separated on an Acclaim PepMap C18 column (75 µm × 150 mm, 3 µm, 100 Å, Thermo, 160321) with a linear gradient (5–90% B for 50 min and 90% B for 5 min, A: 0.1% formic acid, B: 0.1% formic acid and 80% acetonitrile) at a flow rate of 300 nL/min. The separated peptides were online detected in QExative mass spectrometer (Thermo). The raw data were converted into MGF files using MM file conversion. Protein identification was performed using ProteinPilotTM 4.5 (AB Sciex, Framingham, MA, USA) and compared against the putative lncRNA-encoded proteins and UniProt protein database (https://www.uniprot.org/).
To quantify protein, parallel reaction monitoring (PRM) analyses were performed using a QExactive mass spectrometer (Thermo). The PRM method employed an orbitrap resolution of 17,500, a target automatic gain control value of 5e4, and maximum fill times of 120 ms. The PRM proteomics data were analyzed with Skyline (MacCoss, Seattle, WA, USA).
Statistical analysis
All experiments were replicated at least 3 times. Data were represented as the mean ± standard deviation of these replicates. All statistical analyses were performed using SPSS version 19.0 (IBM Corp., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to determine the statistically significant differences among multiple groups. Statistical significance was considered when P<0.05.
Results
TAK-242 alleviated OGD/R-induced injury in BMECs
Given that our previous study suggested that the TLR4 pathway was activated in OGD/R-impaired BMECs (12), the BMECs were exposed to TAK-242, shutting off the TLR4 signaling, during OGD/R just before reoxygenation. As shown in Figure 1A, OGD/R impaired the cell viability of BMECs as expected, and TAK-242 significantly promoted the cell viability of OGD/R cells. Since the TLR4 pathway is well known to regulate the expression of multiple proinflammatory and inflammatory factors, the impact of TAK-242 on OGD/R-induced IL-1β, IL-6, and TNF-α were evaluated. The results of ELISA suggested that TAK-242 suppressed IL-1β, IL-6, and TNF-α inductions in OGD/R cells (Figure 1B-1D).
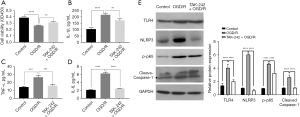
In our previous study (12), OGD/R was demonstrated to stimulate TLR4/NLRP3/Caspase-1 axis and induce pyroptosis in BMECs. To explore the effect of TAK-242 on Caspase-1-dependent pyroptosis, western blot was performed. As shown in Figure 1E, NLRP3 protein and Caspase-1 cleavage in OGD/R cells were remarkably decreased by TAK-242, implying that TAK-242 could alleviate OGD/R-induced Caspase-1-dependent pyroptosis. NF-κB is an important downstream gene of TLR4 signaling and a vital transcription factor that controls the transcription of a variety of pro-inflammatory genes, including IL-1β, IL-6, and TNF-α (37). Our results showed that the phosphorylation of p65 (p-p65) in BMECs was increased after OGD/R treatment, whereas p-p65 in TAK-242+OGD/R was lower than OGD/R cells, indicating that TAK-242 suppressed OGD-R-mediated NF-κB activation (Figure 1E).
TAK-242 altered the lncRNAs expression pattern in OGD/R BMECs
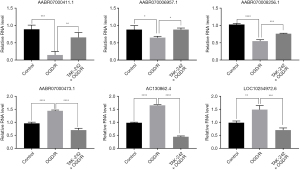
Besides controlling the protein-mediated signaling transduction, TLR4 also regulates lncRNAs expression, contributing to the immune and inflammation responses (24). To investigate the lncRNAs associated with TLR4 during OGD/R, the RNA from the cells was subjected to RNA deep sequencing. The lncRNAs 612, 507, and 628 were respectively identified in control, OGD/R, and TAK-242+OGD/R BMECs, and 413 lncRNAs were commonly expressed in these 3 groups cells (Figure 2A). These commonly expressed lncRNAs were distributed on all of the chromosomes, with chromosome 1 having the most expressed lncRNAs and chromosome 11 having the least expressed lncRNAs (Figure 2B). The length of the lncRNAs was mainly ranged from 200 to 1000 bp (Figure 2C). The differential expression analysis suggested 18 lncRNAs were increased in OGD/R cells (vs. control cells) but decreased in TAK-242+OGD/R cells (vs. OGD/R), and 20 lncRNAs were suppressed in OGD/R cells (vs. control cells) but elevated in TAK-242+OGD/R cells (vs. OGD/R) (Figure 2D). These 38 differently expressed lncRNAs are listed in Table S1. The top 3 increased OGD/R-impaired lncRNAs (AABR07000411.1, AABR070006957.1 and AABR070008256.1) and top 3 decreased OGD/R-induced lncRNAs (AABR07000473.1, AC130862.4 and LOC10254972.6) in TAK-242+OGD/R BMECs were selected and validated using qRT-PCR. Our results confirmed that these 6 lncRNAs were significantly differentially expressed among the groups, and the expression changes were consistent with RNA sequencing data (Figure 3). These results indicated that TAK-242 could restore some OGD/R-impaired lncRNAs and inhibit some OGD/R-induced lncRNAs, implying that TLR4 may regulate the expression of these lncRNAs and that these lncRNAs may be involved in OGD/R injury.

TAK-242 altered the mRNAs expression pattern in OGD/R BMECs
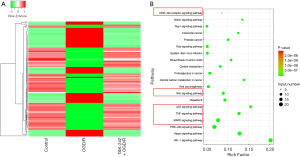
Besides lncRNAs, the mRNAs expression pattern of each group was profiled. Our results suggested that 1,336 genes had opposite expression trends in OGD/R (vs. control) and TAK-242+OGD/R (vs. OGD/R). A total of 632 genes were increased in the OGD/R group versus control group but decreased in the TAK-242+OGD/R group versus OGD/R group; 704 genes were decreased in the OGD/R group versus control group but increased in the TAK-242+OGD/R group versus OGD/R group (Figure 4A and available online: https://cdn.amegroups.cn/static/public/10.21037jtd-23-360-1.pdf). The KEGG pathway analysis indicated that these differently expressed genes were significantly enriched in the pathways linked to inflammatory response, cell fate, and cell proliferation, including NOD-like receptor, Wnt, p53, TNF, and the MAPK signaling pathway (Figure 4B).
ceRNAs regulatory network
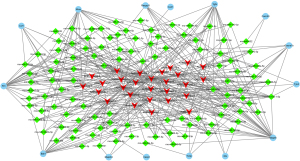
Some lncRNAs have miRNAs binding sites and function as ceRNAs to regulate gene expression. Thus, we analyzed the correlation between the differentially expressed mRNAs and lncRNAs and predicted miRNAs target sites in mRNAs and lncRNAs, in differentially expressed mRNAs. Only those that were involved in inflammatory response, cell fate, and cell proliferation, including NOD-like receptor, Wnt, p65, TNF, and MAPK signaling pathway, were included. Next, a lncRNAs-miRNAs-mRNAs interaction network was constructed (Figure 5). This ceRNA network was composed of 15 mRNAs nodes, 107 miRNAs nodes, and 28 lncRNAs nodes. Notably, AABR07000411.1 interacted with 29 miRNAs and may regulate the expression of 11 genes by ceRNAs mechanism, implying the great role of this lncRNA in OGD/R injury.
Identification of lncRNA-encoded small peptides
As some functional lncRNA-encoded small peptides have been found by several studies (29,30), the protein-coding potential of differently expressed lncRNAs was assessed. The results of LM-MS identified the 133 peptide fragments derived from 59 putative lncRNA-encoded proteins (available online: https://cdn.amegroups.cn/static/public/10.21037jtd-23-360-2.xlsx). And after analysis by Skyline software, 71 peptide fragments derived from 53 putative lncRNA-encoded proteins were further subjected to RPM assay to evaluate proteins expression levels in control, OGD/R, and TAK-242+OGD/R cells (available online: https://cdn.amegroups.cn/static/public/10.21037jtd-23-360-3.xls). The results showed that 12 lncRNA-encoded proteins were confirmed, and 4 of them (AABR07049961.1, AABR07025303.1, AC127076.2, AABR07066020.1) were commonly expressed in control, OGD/R, and TAK-242+OGD/R cells (Figure 6 and Table S2). AABR07049961.1-encoded protein was increased in OGD/R cells versus control but decreased in TAK-242+OGD/R cells versus OGD/R cells (Table S3). AC127076.2 and AABR07066020.1-encoded proteins were decreased in OGD/R cells versus control but were somewhat restored by TAK-242 in OGD/R cells (available online: https://cdn.amegroups.cn/static/public/10.21037jtd-23-360-2.xlsx). AABR07025303.1-encoded protein was decreased in OGD/R cells, and TAK-242 did not affect the expression levels of this protein (Table S3). These results suggested that TLR4 regulated lncRNA-encoded proteins expression during OGD/R, and these small peptides may participate in OGD/R injury.

Discussion
TLR4 is activated by endogenous proteins released from ischemia-damaged tissues, including heat-shock proteins, high mobility group box 1, hyaluronic acid, and fibronectin, among other, that trigger NF-κB activation, leading to the inflammatory response and exacerbation of ischemia injury (18,38-40). Suppression of TLR4 with TAK-242 has been documented to improve ischemic injury in the brain, liver, heart, kidney, and epilepsy (16,33,41-43). In this study, we reported that TAK-242 enhanced cell viability, suppressed pyroptosis, and decreased inflammatory factors release in OGD/R BMECs. The protective effect of TAK-242 against OGD/R-induced injury implied the possibility of adjunctive neuroprotective therapies during DHCA.
RNA deep sequencing was performed to explore the mechanism underlying the TAK-242 protective effect. Our results suggested that OGD/R altered some lncRNAs expression via TLR4 signaling, and TAK-242 could alleviate OGD/R-induced changes in lncRNAs expression pattern. AABR07000411.1 showed the greatest decrease in OGD/R cells in comparison to the control (Figure 3). Meanwhile, TAK-242 almost restored AABR07000411.1 to normal values (Figure 3), hinting that this lncRNA was closely regulated by TLR4 signaling.
We further comprehensively analyzed differently expressed lncRNAs and genes, and plotted lncRNAs-miRNAs-mRNAs networks. In the networks, we identified 15 co-expressed protein-coding genes with lncRNAs via miRNAs (Figure 5), including brain-derived neurotrophic factor (BDNF), Caspase-3 (Casp3), C-X-C motif chemokine ligand 1 (CXCL1), fms-related receptor tyrosine kinase 1 (FLT1), mitogen-activated protein kinase kinase 3 (MAP2K3), mitogen-activated protein kinase 8 (MAPK8), muscle RAS oncogene homolog (MRAS), and transforming growth factor alpha (TGF-α). All of BDNF, MAP2K3, MAPK8, MRAS, and TGF-α are closely connected to cell proliferation, survival, and differentiation (44-47): Casp3 mediates cell apoptosis (48), CXCL1 functions as a chemoattractant for neutrophils and is associated with inflammation (49), and FLT1 plays an important role in angiogenesis and vasculogenesis (50). These results indicated that some TLR4-related lncRNAs may function as ceRNAs to regulate gene expression, and participate in cell survival, inflammation, and angiogenesis during ischemic injury.
In lncRNAs-miRNAs-mRNAs networks, AABR07000411.1 was predicted to regulate 11 protein-coding genes via interacting with 29 miRNAs. Notably, this lncRNA contained 3 miR-130a-5p, 2 miR-20a, and 4 miR-466-3p family binding sites. MiR-130a-5p and miR-20a have been reported to be increased during cerebral ischemia (51,52). Ischemia-induced miR-130a is predominantly from BMECs (51). Suppressing miR-130a could attenuate brain edema, lower BBB permeability, reduce infarct volume, and improve neurologic function in cerebral ischemia/reperfusion model rats (51). Inhibiting miR-20a promoted MAT2B and Bcl-2 expression, while inhibited Bax expression in hypoxic-ischemic brain damage animal model (52). Although research on the role of miR-466-3p in ischemic injury is limited, this miRNA is predicted to have more than 300 putative target genes, and these target genes are involved in many biological processes, indicating the multiple biological functions of miR-466-3p (53). Given that TAK-242 restored the decreased AABR07000411.1 in OGD/R cells, this lncRNA may play a crucial role in the protective effect of TAK-242.
Considering the emerging evidence on lncRNAs-encoded short peptides with biological functions, we explored the protein-coding potential of the differently expressed lncRNAs with LS-MS and assessed the lncRNAs-encoded short peptides expression levels among the control, OGD/R, and TAK-242+OGD/R cells with RPM assay. Our results suggested that AABR07049961.1, AC127076.2, AABR07025303.1, and AABR07066020.1 could encode short peptides, and these short peptides were associated with OGD/R and/or TLR4 signaling. However, these findings come from the rat-origin BMECs, cannot reflect the situation of human. In our future work, we will further confirm these short peptides expression with western blot assay, explore the biological function of these lncRNAs and related key miRNAs in human BMECs.
Conclusions
Taken together, our results suggest that TAK-242 attenuates OGD/R injury in BMECs. TAK-242 inhibits the TLR4/NLRP3/Caspase-1 and TLR4/ NF-κB pathways to decrease OGD/R-induced pyroptosis and inflammation. Moreover, TAK-242 alters lncRNAs expression pattern in OGD/R cells, and the differently expressed lncRNAs may exert a protective effect against OGD/R injury by ceRNA mechanism and encoding short peptides.
Acknowledgments
Funding: This work was supported by the National Natural Science Funds of China (Nos. 81860337, 81960326, 82060384); Natural Science Funds of Jiangxi Province (Nos. 20202ACBL206014, 20192BAB205009, 2020BABL206116); Science & Technology Program of Jiangxi Health Commission (Nos. 20201080, 202130660); National Health Commission Science &Technology Development Research Center (No. 2019ZH-07E-003); general projects of Jiangxi Traditional Chinese Medicine Science and Technology (No. 2020B0214); the Science and Technology Plan Project of Jiangxi Provincial Health Commission (No. 202312165) and the Science and Technology Bureau of Ganzhou City (No. GZ2022ZSF704).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-360/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-360/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-360/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-360/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experimental procedures complied with local and international guidelines for the care and use of animals and were approved by the Animal Ethics Welfare Committee of First Affiliated Hospital of Gannan Medical University (approval No. GFAC-AEWC-121).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alkhatip AAAMM, Kamel MG, Farag EM, et al. Deep Hypothermic Circulatory Arrest in the Pediatric Population Undergoing Cardiac Surgery With Electroencephalography Monitoring: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth 2021;35:2875-88. [Crossref] [PubMed]
- Gocoł R, Hudziak D, Bis J, et al. The Role of Deep Hypothermia in Cardiac Surgery. Int J Environ Res Public Health 2021;18:7061. [Crossref] [PubMed]
- Higo M, Shimizu Y, Wakabayashi K, et al. Post-Operative Kidney Function Using Deep Hypothermic Circulatory Arrest (DHCA) in Aortic Arch Operation. Int J Nephrol Renovasc Dis 2022;15:239-52. [Crossref] [PubMed]
- Amir G, Ramamoorthy C, Riemer RK, et al. Neonatal brain protection and deep hypothermic circulatory arrest: pathophysiology of ischemic neuronal injury and protective strategies. Ann Thorac Surg 2005;80:1955-64. [Crossref] [PubMed]
- Nicolicht-Amorim P, Delgado-Garcia LM, Nakamura TKE, et al. Simple and efficient protocol to isolate and culture brain microvascular endothelial cells from newborn mice. Front Cell Neurosci 2022;16:949412. [Crossref] [PubMed]
- Engelhardt S, Huang SF, Patkar S, et al. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: a comparative study. Fluids Barriers CNS 2015;12:4. [Crossref] [PubMed]
- Wang DP, Kang K, Sun J, et al. URB597 and Andrographolide Improve Brain Microvascular Endothelial Cell Permeability and Apoptosis by Reducing Oxidative Stress and Inflammation Associated with Activation of Nrf2 Signaling in Oxygen-Glucose Deprivation. Oxid Med Cell Longev 2022;2022:4139330. [Crossref] [PubMed]
- Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B 2015;5:8-24. [Crossref] [PubMed]
- Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol 2000;10:113-26. [Crossref] [PubMed]
- Zhang M, Wu Q, Tang M, et al. Exosomal Mir-3613-3p derived from oxygen-glucose deprivation-treated brain microvascular endothelial cell promotes microglial M1 polarization. Cell Mol Biol Lett 2023;28:18. [Crossref] [PubMed]
- Wu KW, Kou ZW, Mo JL, et al. Neurovascular coupling protects neurons against hypoxic injury via inhibition of potassium currents by generation of nitric oxide in direct neuron and endothelium cocultures. Neuroscience 2016;334:275-82. [Crossref] [PubMed]
- Kong LY, Liang MY, Liu JP, et al. Mesenchymal Stem Cell-derived Exosomes Rescue Oxygen-Glucose Deprivation-induced Injury in Endothelial Cells. Curr Neurovasc Res 2020;17:155-63. [Crossref] [PubMed]
- Kong LY, Li Y, Rao DY, et al. miR-666-3p Mediates the Protective Effects of Mesenchymal Stem Cell-derived Exosomes Against Oxygen-glucose Deprivation and Reoxygenation- induced Cell Injury in Brain Microvascular Endothelial Cells via Mitogen-activated Protein Kinase Pathway. Curr Neurovasc Res 2021;18:20-77. [Crossref] [PubMed]
- Elloumi N, Tahri S, Fakhfakh R, et al. Role of innate immune receptors TLR4 and TLR2 polymorphisms in systemic lupus erythematosus susceptibility. Ann Hum Genet 2022;86:137-44. [Crossref] [PubMed]
- Jin S, Nepal N, Gao Y. The role of toll-like receptors in peptic ulcer disease. Immunol Med 2022;45:69-78. [Crossref] [PubMed]
- Dong J, Liao Y, Wu B. TAK-242 ameliorates epileptic symptoms in mice by inhibiting the TLR4/NF-κB signaling pathway. Ann Transl Med 2022;10:795. [Crossref] [PubMed]
- Cui Y, Zhang NN, Wang D, et al. Modified Citrus Pectin Alleviates Cerebral Ischemia/Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation via TLR4/NF-ĸB Signaling Pathway in Microglia. J Inflamm Res 2022;15:3369-85. [Crossref] [PubMed]
- Zhao H, Chen Z, Xie LJ, et al. Suppression of TLR4/NF-κB Signaling Pathway Improves Cerebral Ischemia-Reperfusion Injury in Rats. Mol Neurobiol 2018;55:4311-9. [Crossref] [PubMed]
- Lai JL, Liu YH, Liu C, et al. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017;40:1-12. [Crossref] [PubMed]
- Black KE, Collins SL, Hagan RS, et al. Hyaluronan fragments induce IFNβ via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway. J Inflamm (Lond) 2013;10:23. [Crossref] [PubMed]
- Zhou X, Quan H, Zang L, et al. Dichotomine B Attenuates Neuroinflammatory Responses by Regulating TLR4/MyD88-mTOR Signaling Pathway in BV2 Cells. Neurochem Res 2023; Epub ahead of print. [Crossref]
- Sideris N, Dama P, Bayraktar S, et al. LncRNAs in breast cancer: a link to future approaches. Cancer Gene Ther 2022;29:1866-77. [Crossref] [PubMed]
- Ran R, Gong CY, Wang ZQ, et al. Long non-coding RNA PART1: dual role in cancer. Hum Cell 2022;35:1364-74. [Crossref] [PubMed]
- Xu S, Wang J, Jiang J, et al. TLR4 promotes microglial pyroptosis via lncRNA-F630028O10Rik by activating PI3K/AKT pathway after spinal cord injury. Cell Death Dis 2020;11:693. [Crossref] [PubMed]
- Atianand MK, Hu W, Satpathy AT, et al. A Long Noncoding RNA lincRNA-EPS Acts as a Transcriptional Brake to Restrain Inflammation. Cell 2016;165:1672-85. [Crossref] [PubMed]
- Zhang J, Zheng Y, Xu J. Editorial: Computational Identification of ceRNA Regulation. Front Mol Biosci 2022;9:937505. [Crossref] [PubMed]
- Yin R, Qiu C, Shen Q, et al. Corin is regulated by circ-0012397/miR-200a-3p and inhibits the oxygen-glucose deprivation-induced apoptosis of SHSY5Y neuroblastoma cells. Ann Transl Med 2022;10:1242. [Crossref] [PubMed]
- Xue ST, Zheng B, Cao SQ, et al. Long non-coding RNA LINC00680 functions as a ceRNA to promote esophageal squamous cell carcinoma progression through the miR-423-5p/PAK6 axis. Mol Cancer 2022;21:69. [Crossref] [PubMed]
- Bukhari I, Khan MR, Hussain MA, et al. PINTology: A short history of the lncRNA LINC-PINT in different diseases. Wiley Interdiscip Rev RNA 2022;13:e1705. [Crossref] [PubMed]
- Zhang Y, Wang X, Hu C, et al. Shiny transcriptional junk: lncRNA-derived peptides in cancers and immune responses. Life Sci 2023;316:121434. [Crossref] [PubMed]
- Yu J, Wang W, Yang J, et al. LncRNA PSR Regulates Vascular Remodeling Through Encoding a Novel Protein Arteridin. Circ Res 2022;131:768-87. [Crossref] [PubMed]
- Ii M, Matsunaga N, Hazeki K, et al. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol 2006;69:1288-95. [Crossref] [PubMed]
- Fujiwara M, Matoba T, Koga JI, et al. Nanoparticle incorporating Toll-like receptor 4 inhibitor attenuates myocardial ischaemia-reperfusion injury by inhibiting monocyte-mediated inflammation in mice. Cardiovasc Res 2019;115:1244-55. [Crossref] [PubMed]
- Yokoi T, Yokoyama Y, Kokuryo T, et al. Inhibition of Toll-like receptor 4 ameliorates experimental postischemic injury in the cholestatic liver through inhibition of high-mobility group box protein b1 (HMGB1) signaling. Surgery 2018;163:270-6. [Crossref] [PubMed]
- Rice TW, Wheeler AP, Bernard GR, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med 2010;38:1685-94. [Crossref] [PubMed]
- Abdul Y, Abdelsaid M, Li W, et al. Inhibition of Toll-Like Receptor-4 (TLR-4) Improves Neurobehavioral Outcomes After Acute Ischemic Stroke in Diabetic Rats: Possible Role of Vascular Endothelial TLR-4. Mol Neurobiol 2019;56:1607-17. [Crossref] [PubMed]
- Li J, Wang L, Zeng G, et al. Chymotrypsin attenuates adjuvant-induced arthritis by downregulating TLR4, NF-κB, MMP-1, TNF-α, IL-1β, and IL-6 expression in Sprague-Dawley rats. Immunopharmacol Immunotoxicol 2022;44:959-69. [Crossref] [PubMed]
- Yuan C, Chen Z, Zhou Q. Crocin inhibits KBTBD7 to prevent excessive inflammation and cardiac dysfunction following myocardial infarction. Mol Med Rep 2023;27:20. [Crossref] [PubMed]
- Liu J, Zhang S, Fan X, et al. Dexmedetomidine Preconditioning Ameliorates Inflammation and Blood-Spinal Cord Barrier Damage After Spinal Cord Ischemia-Reperfusion Injury by Down-Regulation High Mobility Group Box 1-Toll-Like Receptor 4-Nuclear Factor κB Signaling Pathway. Spine (Phila Pa 1976) 2019;44:E74-81. [Crossref] [PubMed]
- Zhang YL, Li PB, Han X, et al. Blockage of Fibronectin 1 Ameliorates Myocardial Ischemia/Reperfusion Injury in Association with Activation of AMP-LKB1-AMPK Signaling Pathway. Oxid Med Cell Longev 2022;2022:6196173. [Crossref] [PubMed]
- Zhu K, Zhu X, Sun S, et al. Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp Neurol 2021;345:113828. [Crossref] [PubMed]
- Zhong X, Xiao Q, Liu Z, et al. TAK242 suppresses the TLR4 signaling pathway and ameliorates DCD liver IRI in rats. Mol Med Rep 2019;20:2101-10. [Crossref] [PubMed]
- Mohammad BI, Raheem AK, Hadi NR, et al. Reno-protective effects of TAK-242 on acute kidney injury in a rat model. Biochem Biophys Res Commun 2018;503:304-8. [Crossref] [PubMed]
- Di Carlo P, Punzi G, Ursini G. Brain-derived neurotrophic factor and schizophrenia. Psychiatr Genet 2019;29:200-10. [Crossref] [PubMed]
- Sun Y, Liu WZ, Liu T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 2015;35:600-4. [Crossref] [PubMed]
- Kimmelman AC, Osada M, Chan AM. R-Ras3, a brain-specific Ras-related protein, activates Akt and promotes cell survival in PC12 cells. Oncogene 2000;19:2014-22. [Crossref] [PubMed]
- Kumar V, Bustin SA, McKay IA. Transforming growth factor alpha. Cell Biol Int 1995;19:373-88. [Crossref] [PubMed]
- Asadi M, Taghizadeh S, Kaviani E, et al. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol Appl Biochem 2022;69:1633-45. [Crossref] [PubMed]
- Wang S, Bai J, Zhang YL, et al. CXCL1-CXCR2 signalling mediates hypertensive retinopathy by inducing macrophage infiltration. Redox Biol 2022;56:102438. [Crossref] [PubMed]
- Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res 2004;117:3-32. [Crossref] [PubMed]
- Wang Y, Wang MD, Xia YP, et al. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J 2018;32:935-44. [Crossref] [PubMed]
- He H, Sun M, Chen Y, et al. Dexmedetomidine alleviates the hypoxic-ischemic brain damage via miR-20a-5p/methionine adenosyltransferase 2B axis in rat pups. Neuroreport 2022;33:205-14. [Crossref] [PubMed]
- Akimniyazova A, Pyrkova A, Uversky V, et al. Predicting Associations of miRNAs and Candidate Gastric Cancer Genes for Nanomedicine. Nanomaterials (Basel) 2021;11:691. [Crossref] [PubMed]


