Delayed iatrogenic diaphragmatic hernia after thoracoscopic lobectomy
Introduction
Iatrogenic diaphragmatic hernia is rare complications of thoracic or abdominal surgery, having been described following esophagectomy (1), gastrectomy (2), laparoscopic cholecystectomy (3), and nephrectomy (4). An acute presentation with a strangulated or an obstructed viscus may present a diagnostic dilemma and require urgent resection and repair. Herein, we describe an extremely rare complication of diaphragmatic hernia that occurring in a woman several months after VATS left upper lobectomy with lymphadenectomy.
Case presentation
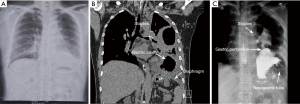
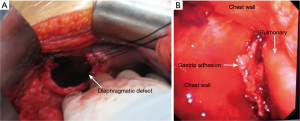
A 55-year-old woman (written informed consent was obtained) was referred to our hospital for complaining of intermittent nausea, vomiting and distension on 10 Dec 2014. Physical examination revealed a weakness of breath sounds in the left thorax. The abdominal examination was negative. Past history revealed that she had undergone a left upper lobectomy with systematic mediastinal lymphadenectomy for lung cancer. It was performed by completely thoracoscopic approach in local hospital on 21 July 2014. She was discharged on the eighth postoperative day without any morbid conditions. The pathological stage of lung cancer was IA (T1aN0M0, Adenocarcinoma). Three months after the surgery, she was received chest radiography (Figure 1A) in local hospital. A nonspecific postoperative sign which may indicate the left pleural effusion or any other abnormalities was showed. As no complaining of gastrointestinal discomforts or dyspnea, the patient refused to undergo further examinations. Furthermore, there was no history of any type of abdominal or thoracic trauma during this intervening period. During the rehospitalization, nasogastric tube was inserted, which alleviated her symptoms promptly. Initially, chest computed tomography (CT) was arranged which indicated the diaphragmatic defect with gastric hernia (Figure 1B). The diagnosis of diaphragmatic hernia with gastric perforation was confirmed by barium swallow (Figure 1C). Therefore, urgent laparotomy was scheduled. Intraoperatively, the stomach was incarcerated in the chest through the diaphragmatic defect (Figure 2A). The thoracic cavity was also inspected closely under the choledochoscopy which was inserted through the diaphragmatic defect. Due to the secondary inflammation of perforation, the adhesion which located between the lesser curvature of stomach and thorax was carefully dissected. Meanwhile, the thoracic hemostasis was verified using by ultrasonic activated scissor under the choledochoscopy (Figure 2B). Subsequently, the stomach was gently reduced into the abdominal cavity. On account of gastric gangrene and secondary perforation, partial stomach was needed to be resected and nasojejunal tube was placed as well. Thereafter, the diaphragmatic defect was repaired with non-absorbable interrupted sutures. The postoperative recovery of the second surgery was uneventfully. Up to present, the patient was still well and no evidence of cancer recurrence was detected.
Discussion
Iatrogenic diaphragmatic hernia is a rare complication of thoracic and abdominal surgery (5). The low incidence of iatrogenic diaphragmatic hernia might be the result of a high misdiagnosis rate. Though the rare occurrence of iatrogenic diaphragmatic hernia, the mortality following emergency surgery for strangulated or perforated bowel or stomach rises to between 20% and 80% (6). To our knowledge, no document was found to describing the delayed complication several months after VATS left upper lobe (LUL) lobectomy. In this case, the left lower lobe (LLL) was completely adhered to diaphragm and the adhesions had been dissected with ultrasonic scissor in the first surgery. After reviewing the initial surgical procedure which may lead to diaphragmatic hernia, we suppose that a weak point (or an imperceptible perforation) in the diaphragm might have been provoked by the ultrasonic activated scissor during the LLL-diaphragmatic adhesiolysis. Though, it may not be observed during the first operation. Furthermore, the continuous suction of chest drainage used in the initial surgical procedure which might amplify transabdominal-pleural cavity pressure. Any small defect in the diaphragm is likely to increase in size as a result of the gradient of pressure between the abdominal and pleural cavities. Theoretically, those potential conditions are probably contributive to diaphragmatic hernia progressively, even allowing a migration of the stomach (1,2). However, the herniation of abdominal content may be reducible at the preliminary stage which might not be detected by chest radiography. After incarceration in the thorax, gastrointestinal symptoms such as distension, nausea and vomiting may occur, even the secondary gastric perforation which might result from persistent strangulation of the diaphragmatic hernia against gastric blood vessels. Despite of hemidiaphragmatic eventration after lobectomy, the elevated stomach is not distinctly present under the diaphragm. It needs to be clarified whether or not the stomach has entirely migrated into thorax though the diaphragmatic defect, especially in the patient who had previously received thoracic or abdominal surgery.
Needless to say, a thoracoabdominal approach could have been used as a most reliable approach for managing diaphragmatic hernia (1). Considering with the possibility of intrathoracic adhesions and the feasibility of management of the gastric perforation, we chose the transabdominal approach in this case. Additionally, the choledochoscopy was introduced into left thoracic cavity through the diaphragmatic defect. Owing to its flexibility and narrow width of infusion channel, the choledochoscopy might illuminate operative field much better than rigid endoscope. It was therefore beneficial to obtain precise dissection, satisfactory hemostasis in the thorax and safely reduce the herniated contents to the abdomen. By using the choledochoscopy, it was clearly confirmed that the lower lobe was completely adhered with chest wall without any potentially serious disorders, such as pulmonary abscess or bronchopleural fistula. Therefore, it was unnecessary to release the pleural adhesions or make a conversion to thoracoabdominal route. Herein, compared with solely trans-thoracotomy, transabdominal and thoracoabdominal approach, our surgical approach seems to be a feasible and alternative option for the patients who have been diagnosed as delayed diaphragmatic hernia concomitant with remarkable intra-abdominal findings and have a history of thoracic surgery. This surgical procedure avoiding thoracotomy may have the potential benefits of decreasing postoperative mechanical ventilation, pulmonary complications, length of stay (1).
To decreasing the incidence of iatrogenic diaphragmatic hernia after surgery, it is important that surgeons should inspect the integrity of diaphragm in the end of surgery. Indeed, a good number of experiences with ultrasonic activated scissor would be the effective measures to avoid this complication. During the postoperative follow-up, attention should be paid to abdominal pain, dyspnea, and distension. In conclusion, we should be aware of the possibility of this rare complication in patients presenting with aforementioned symptoms after VATS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Paul S, Nasar A, Port JL, et al. Comparative analysis of diaphragmatic hernia repair outcomes using the nationwide inpatient sample database. Arch Surg 2012;147:607-12. [Crossref] [PubMed]
- Suh Y, Lee JH, Jeon H, et al. Late onset iatrogenic diaphragmatic hernia after laparoscopy-assisted total gastrectomy for gastric cancer. J Gastric Cancer 2012;12:49-52. [Crossref] [PubMed]
- Armstrong PA, Miller SF, Brown GR. Diaphragmatic hernia seen as a late complication of laparoscopic cholecystectomy. Surg Endosc 1999;13:817-8. [Crossref] [PubMed]
- de Meijer VE, Vles WJ, Kats E, et al. Iatrogenic diaphragmatic hernia complicating nephrectomy: top-down or bottom-up? Hernia 2008;12:655-8. [Crossref] [PubMed]
- Axon PR, Whatling PJ, Dwerryhouse S, et al. Strangulated iatrogenic diaphragmatic hernia: a late diagnosed complication. Eur J Cardiothorac Surg 1995;9:664-6. [Crossref] [PubMed]
- Tarver RD. Interventional chest procedures and diaphragmatic hernias. Curr Opin Radiol 1990;2:368-73. [PubMed]


