Diagnostic value of antineutrophil cytoplasmic antibodies in children with bronchiolitis obliterans
Introduction
Bronchiolitis obliterans (BO), a chronic inflammatory process which involves the small airways and typically leads to progressive obliteration of the bronchioles (1), is noted among patients with severe infection, acute lung injury, autoimmune diseases, or in recipients of allogeneic transplantation (2,3). About 1% of children with infectious bronchiolitis may develop BO as a result of microbial respiratory infections (4-9). Unfortunately, BO in pediatric settings is often misdiagnosed as asthma, classical pneumonia or infectious bronchiolitis. This may be partly due to the non-specific clinical signs; moreover, current diagnostic tools show limitations in identifying BO. Briefly, chest radiography usually fails to reveal the lesions; high-resolution computed tomography (HRCT), with the greatest sensitivity and specificity (4,10) in imaging studies for BO, offers only circumstantial evidence of bronchiolar disease, whereas these evidence (such as typical mosaic pattern) share similarities to many other bronchiole-involving disorders (6,11,12); routine bronchoscopy is more useful for exclusive than for confirmatory diagnosis; pulmonary function tests are difficult to performed, and are merely for clinical reference in very young children aged below 5 (13); lung biopsy as the gold standard has a fairly low yield, as the specimens collected from patchy BO lesions are not always adequate (14). In addition, parents and children rarely prefer or comply to these invasive procedures. Repeated lung biopsy for follow-up in BO children can be thus more difficult. There is a dire need for a novel auxiliary approach which is diagnostically valuable and convenient in BO.
Antineutrophil cytoplasmic antibodies (ANCAs) are autoantibodies against components of neutrophils and monocytes (15). Two of these family members, the ANCAs against myeloperoxidase (MPO-ANCA) and proteinase-3 (PR3-ANCA), have been studied in primary vasculitis and autoimmune rheumatic diseases (16,17). In fact, recent research has linked ANCAs to a broad range of conditions, including autoimmune hepatopathy, Kawasaki disease, inflammatory bowel disease, and connective tissue diseases (18,19). In pulmonology, ANCAs have been reported detectable among patients with diffuse interstitial lung disease (20,21). In an uncontrolled study, we noted positive serum MPO- and PR3-ANCAs in 52.6% and 42.1%, respectively, of 19 patients with BO (22).
Therefore we hypothesized that MPO- and PR3-ANCAs may be useful for facilitating diagnosis of BO. If with acceptable accuracy or specificity, serological tests could be clinically important in terms of patient convenience and non-invasiveness. Here we presented a preliminary study on their serological profiles in BO and whether they differ from the findings in bronchial pneumonia.
Methods
Study population
This was a prospective, observational study conducted between June 2009 and December 2013. During the study period, a total of 58 consecutive BO children (45 boys and 13 girls) treated in a tertiary teaching hospital in southern China, the First Affiliated Hospital of Guangzhou Medical University, were approached. The diagnostic criteria of BO were described elsewhere (23): (I) recurrent or persistent wheezing, dyspnea or coughing, and stridors lasting for >6 weeks after acute lower respiratory infection or acute lung injury, with no response to bronchodilators; (II) presence of clinical features inconsistent with the radiography findings, showing more severe symptoms in contrast to signs of hyperventilation on chest radiography; (III) mosaic perfusion pattern, bronchial wall thickening, bronchiectasis or atelectasis on chest HRCT; (IV) pulmonary function tests indicating obstructive ventilatory impairment; and (V) exclusion of other obstructive airway disorders that could lead to wheezing (such as asthma, primary ciliary dyskinesia, cystic fibrosis, foreign bodies, congenital abnormalities, tuberculosis), AIDS and other immune disorders (such as systemic lupus erythematosus and systemic vasculitis). For a rigorous confirmation of BO, in final analysis we included only those children who also fulfilled five of the following: (I) no response to regular inhaled corticosteroids and bronchodilators for ≥12 weeks; (II) negative bronchoscopic findings for airway malformations and tuberculosis; (III) retained exercise intolerance, and mosaic pattern on HRCT despite systemic corticosteroids therapy; (IV) absence of impaired renal function, such as microscopic hematuria or proteinuria; and (V) negative findings for antinuclear antibodies such as anti-Ro(SSA), anti-La(SSB), anti-Sm, or anti-dsDNA antibodies.
A contemporary cohort of 60 children (34 boys and 26 girls) with mild acute bronchial pneumonia was also approached as potential controls. The final inclusion was based on diagnostic criteria of mild acute bronchial pneumonia by Workgroup of Respiratory Diseases, Chinese Medical Association Society of Pediatrics (24), plus all of the following: (I) duration of symptoms <2 weeks; (II) no wheezing, no signs of lobar pneumonia, or severe pneumonia as manifested by hypoxemia and heart failure; (III) negative history for preterm birth, eczema, allergic rhinitis, scar formation, mechanical ventilation, and recurrent respiratory infections; (IV) no family history for allergies and keloidosis; (V) no medications with systemic corticosteroids during the present hospitalization.
For these subjects, we recorded data on their demographics, treatments, length of hospital stay, and serum MPO-ANCA and PR3-ANCA (see below).
Ethics statement
This study was approved by the Ethics Committee of First Affiliated Hospital of Guangzhou Medical University (No. of Approval: GYFYY 2009-05-28). All experiments were performed in accordance with relevant guidelines and regulations of the Ethics Committee of First Affiliated Hospital of Guangzhou Medical University. Written informed consent was obtained from the legal guardians of all participants.
Measurement of serum MPO- and PR3-ANCAs
From each subject, 3 mL venous blood sample was collected on the first day (baseline) and the last day of hospitalization (on discharge). These samples were assigned unique identification numbers before transferring for measurement at the Central Laboratory of our institution. The technical team who completed the serological tests was blinded about patient information, including the diagnosis.
The serum levels of MPO- and PR3-ANCAs were measured by using MPO and PR3 ELISA kits (ZEUS Scientific, Branchburg, New Jersey, USA), respectively, according to the manufacturer’s instructions. Briefly, 100 µL of 1:21 diluted serum sample was added into reaction wells, followed by 30 min incubation at 25 degree Celsius (°C). After five washes, 100 µL of antibody-enzyme conjugate was added, and the plate was incubated for another 30 min at 25 °C. After three washes, 100 µL of substrate was added into each well and, following 15-min incubation at 25 °C, 50 µL of stop solution was added. Finally, the optical density at 450 nm (OD) for each well was measured by using a microplate reader (ThermoFisher Scientific, Shanghai, China). The levels of MPO- and PR3-ANCAs were calculated based on the formula: Test Specimen ANCA (AAU/mL) = Test Specimen OD × Calibrator Unit Value/Calibrator OD. For either MPO-ANCA or PR3-ANCA, the cut-off value for positive test was ≥180 AAU/mL, according to manufacturer’s recommendation.
Statistical analysis
Data were analyzed with the Statistical Package for the Social Sciences version 13.0 (SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used for evaluating the data normality. Normally distributed values were presented as mean ± standard deviation (SD), non-normally distributed values as median and interquartile range (IQR), and the range of reference values as 95% confidence intervals (95% CI). Normally distributed data were compared by using an independent t-test, otherwise by using a rank-sum test for independent samples. Categorical data were expressed with rate or proportion, and analyzed with Chi-squared test or Fisher’s exact test where appropriate. A P value <0.05 was considered significant.
Results
General demographics and clinical characteristics of the study population
There were 42 BO children (BO group) and 43 with mild acute bronchial pneumonia (Pneumonia group) included in the final analysis, while the remaining 33 were excluded because of incompatibility with the inclusion criteria or additional constraints. The general demographics and clinical characteristics of children in both groups are shown in Table 1. According to the timing of sample collection, the median interval between two serum tests in either group was the median length of hospital stay.
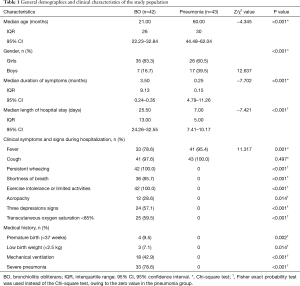
Full table
For the treatments during hospitalization, the both groups received antibiotics, expectorants, and salbutamol. In addition, the BO children received empirical small-dose azithromycin (3 to 5 mg/kg/d) and oral prednisone (1.5 to 2.0 mg/kg/d) or IV methylprednisolone equivalent.
Serological profiles of MPO-ANCA in bronchiolitis obliterans versus mild acute pneumonia
There were more children in the BO group with positive findings for serum MPO-ANCA at baseline [29 (69.1%) vs. 4 (9.3%), χ2=75.662, P<0.001] and on discharge [26 (61.9%) vs. 4 (9.3%), χ2=61.339, P<0.001], respectively, compared with the pneumonia group (Table 2, Figure 1A,B). At baseline, the median level of serum MPO-ANCA in BO children was 292.00 vs. 104.75 AAU/mL in children with pneumonia (Z=3.586, P<0.001); these figures were 310.50 vs. 95.42 AAU/mL on discharge (Z=3.498, P<0.001).
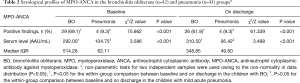
Full table
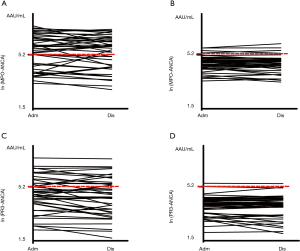
Within-group comparisons did not show statistical differences in the overall rate of positivity and serum level of MPO-ANCA between baseline and on discharge either in BO or in the pneumonia group (both P>0.05).
Serological profiles of PR3-ANCA in bronchiolitis obliterans versus mild acute pneumonia
Similarly, there were more BO children with positive findings for serum PR3-ANCA at baseline [16 (38.1%) vs. 2 (4.7%), χ2=32.262, P<0.001] and on discharge [15 (35.7%) vs. 1 (2.3%), χ2=37.557, P<0.001], respectively, compared with the pneumonia group (Table 3, Figure 1C,D). At baseline, the median level of serum PR3-ANCA in BO children was 106.66 vs. 54.56 AAU/mL in those with pneumonia (Z=3.982, P<0.001); these figures were 97.98 vs. 57.23 AAU/mL on discharge (Z=2.888, P<0.001). Again, within-group comparisons did not show statistical differences in the overall rate of positivity and serum level of PR3-ANCA between baseline and on discharge either in BO or in the pneumonia group (both P>0.05).
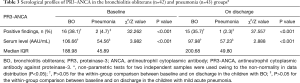
Full table
Single versus dual positivity or negativity to the antineutrophil cytoplasmic antibodies (ANCAs) in bronchiolitis obliterans versus mild acute pneumonia
In the BO group, single-positivity to MPO-ANCA alone was found in 13 children (31.0%) at baseline and 11 (26.2%) on discharge, compared with very few such patients in the pneumonia group [3 (7.0%) at baseline, χ2=18.713, P=0.004; 4 (9.3%) on discharge, χ2=10.009, P=0.002] (Table 4). None of BO children were single-positive to PR3-ANCA alone; in contrast, in the pneumonia group, PR3-ANCA single-positivity was found in one child (2.3%) at baseline and one (2.3%, not the same child as identified at baseline) on discharge, although there was no difference in PR3-ANCA single positivity between the BO and pneumonic children (P=0.497).

Full table
At baseline, there were a remarkably higher rate of dual-positivity to MPO- and PR3-ANCAs [16 (38.1%) vs. 1 (2.3%), χ2=40.500, P<0.001], and a lower rate of dual-negativity to both ANCAs [13 (31.0%) vs. 38 (88.4%), χ2=57.473, P<0.001] in the BO group compared with the pneumonic children. On discharge, 15 children (35.7%) in the BO group were dual-positive to both ANCAs, compared to none in the pneumonia group (Table 4); and the rate of dual-negativity to both ANCAs remained to be lower in the BO group [16 (38.1%) vs. 38 (88.4%), χ2=53.625, P<0.001].
Discussion
Pathologically confirmed BO cases were first reported in 1901 (25). In 1973, 52 more cases of BO were described by Gosink (26). With the advances in diagnostics, increasing patients with BO have been identified over the past decades. Unfortunately, the diagnosis of childhood BO remains difficult owing to various technical setbacks (4,10,11,14), or hampered by the invasiveness and poor patient compliance. In a study by Colom (27), the diagnosis of BO in 120 children ineligible for lung biopsy due to young age, airflow limitation or other reasons relied alternatively on clinical scoring and a 10-year follow-up. Serological tests of biomarkers could thus be clinically useful as a convenient approach for diagnosis. However, such biomarkers in BO are yet to be established.
Recently, the clinical value of ANCAs has been discussed in diseases ranging from glomerulonephritis, vasculitis to ulcerative colitis (16-19,22,28). Studies on ANCAs were driven by the putative endothelial damage by massive release of oxygen free radicals and proteolytic enzymes owing to ANCA-induced neutrophil activation under inflammatory conditions, although the exact mechanism remains to be clarified (29,30). Following this logic, ANCAs may also be involved in chronic lung diseases [such as interstitial lung disease (20,21)] where airway neutrophilia is frequently present and considered to correlate with disease severity (31). Studies have shown high neutrophil counts in the bronchoalveolar lavage fluid (BALF) of patients with BO after lung transplantation, but not in those without BO, which might be explained by long-term neutrophil colonization in the lungs (32). Among measles patients who subsequently developed BO, there was also an increase in BALF neutrophils compared with controls (7).
In 2012, we noted a high positive rate of ANCAs among 19 BO children with a history of severe pulmonary infection, although that cohort was small and did not include any control group (22). Unlike in adults, childhood BO is related more to severe, recurrent respiratory infections (6,7,33,34). Serum ANCAs may also be associated with various microbial infections (4-9). Given these, we recruited children with mild acute pneumonia as controls. Our selection criteria for the pneumonia group were rigorously designed to exclude asthma, allergies, scarring and other conditions that might complicate our results.
The present study showed significantly higher positive rates and serum levels of MPO- and PR3-ANCAs in the BO group compared with the pneumonia group, indicating an association between ANCAs and BO. Our study was not designed to elucidate on the mechanism underlying this observation. However, based on findings for ANCA in other diseases (35,36), we speculated that ANCAs in these BO patients might have activated neutrophils to release large amounts of oxygen free radicals and proteolytic enzymes, which in turn caused epithelial detachment and necrosis in the small airways, and resulted in abnormal tissue repair, submucosal fibrosis, hyalinization, and collagen deposition. Subsequently, the fibrosis and scarring could lead to narrowing, distortion and occlusion of the bronchiolar lumen.
It would be important to note that environmental factors (such as silica dusts), bacterial or viral infections, drugs and genetic susceptibility may interact to affect the positive findings for ANCAs (37). In the present study with rigorous inclusion criteria to minimize confounding factors, MPO-ANCA was detected in >60%, and PR3-ANCA in >35%, respectively, of the BO children. These positive findings persisted over a median duration of 25.50 days, and were mostly noted in children who were dual-positive to MPO- and PR3-ANCA (38.1% at baseline, 35.7% on discharge). In contrast, the pneumonia group showed much lower positive rates of MPO-ANCA (<9.5%) or PR3-ANCA (<5.0%) either at baseline or on discharge (a shorter median of 7.00 days later); and according to our observation, the positivity was more likely to be single-positivity to MPO- or PR3-ANCA alone, and much less frequently to be dual-positivity to the both (Table 4). The serum ANCAs levels in these single-positive children (n=4 at baseline and n=5 on discharge) (Table 4) were considerably low, noted to be near although modestly above the cut-off value (180 AAU/mL). In addition, the majority of ANCA single-positive pneumonic children differed in patient identification between the baseline and on discharge (Figure 1B,D). These observations suggested two probable reasons: acute infections might also contribute to transient positivity of ANCAs or, the single-positive findings in pneumonic children may arise from systematic error of the testing in our study. We expect that future studies could be elaborated to further minimize the confounding effect of acute infections and improve the diagnostic potential of serum ANCAs in BO.
A positive correlation between ANCAs and disease activity has been reported in primary vasculitis, where serum ANCA levels decreased or diminished in patients who responded to treatment but increased in those who relapsed (38,39). Therefore these authors proposed using ANCAs as a biomarker for diagnosing ANCA-associated vasculitis, evaluating disease severity, and predicting prognosis. In the present study, the serum MPO- and PR3-ANCA levels were high in BO children at baseline, and remained so over a longer duration of hospital stay. Interestingly, ANCAs as autoimmune antibodies are mainly IgG with a half-life of ~21 days. Thus, studies with longer period to follow up the serum ANCAs in these BO children should be of interest, and in fact, our ongoing study is now looking at this until the end of 2016 (≥3 years after the first measurement). Since ANCA production might be activated by MPO and PR3 antigens on the surface of neutrophils that colonize the pulmonary tissues as a result of small airway obstruction, recurrent inflammation and infections, we speculated that even after short-term anti-inflammatory and antimicrobial treatments, the serum ANCA levels would remain elevated until the airway neutrophilic infiltration is substantially improved. Compared with acute infections like simple pneumonia, the airway neutrophilic infiltration can be much more severe in BO, and hence probably the longer duration and greater amplitude of elevated serum MPO- and PR3-ANCAs as found in the present study.
We should acknowledge inadequate basic research on serum ANCAs in BO, the small sample size in our study, and the presence of ANCAs in many other disorders, may prevent us from recommending ANCA as a specific biomarker of BO. Nonetheless, the present study may be an early attempt to shed light on serum ANCA profiles in BO. When interpreted in the context of clinical signs, medical history and imaging findings, serum ANCA tests may become a convenient auxiliary approach for diagnosis of BO. In particular, dual-positivity to the two ANCAs seemed to offer specificity in determining BO. For single-positivity to ANCA, we speculated that using a higher cut-off value (220 AAU/mL) instead of 180 AAU/mL, would also yield higher specificity in the diagnosis (Figure 1), although this needs further clarification in larger studies.
There were several limitations in the present study. Firstly, none case of BO in this study was diagnosed based on open lung biopsy. This could potentially result in a selection bias. We attempted to circumvent this bias with rigorous constraints in patient inclusion. At the time of writing, these BO patients are still in follow-up. Secondly, the mean age and the gender distribution were not comparable between the with BO and pneumonia groups. While this mis-match is to be controlled in future studies, to the best of our knowledge, there is little evidence in the literature suggesting patient age and gender may interfere with serum ANCA levels. Thirdly, a healthy control group was not present because this was an observational study based on clinical practice, and that serum ANCA tests in healthy subjects were not part of a regular health checkup. Fourthly, follow-up data on MPO- and PR3-ANCAs in the BO children were not available, because the follow-up is ongoing until the end of 2016. Future studies are therefore needed to address these limitations, validate our findings, and further clarify the clinical value of ANCAs in BO.
Acknowledgements
The authors are grateful to Ms. Mei Jiang for her generous assistance in medical statistics, and to Kangni Chen who edited the format of the text. Special gratitude goes to the technical team at Central Laboratory, First Affiliated Hospital of Guangzhou Medical University, for their work in serological tests.
Funding: Guangdong Natural Science Foundation (Project No. S2012010008784); Guangdong Provincial Science and Technology Project (Project No. 2014A020212356); Guangzhou Municipal Science and Technology Innovation Project (Project No. 201504281719217).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Colby TV. Bronchiolitis. Pathologic considerations. Am J Clin Pathol 1998;109:101-9. [Crossref] [PubMed]
- Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med 2014;370:1820-8. [Crossref] [PubMed]
- Lynch JP 3rd, Weigt SS, DerHovanessian A, et al. Obliterative (constrictive) bronchiolitis. Semin Respir Crit Care Med 2012;33:509-32. [Crossref] [PubMed]
- Kim CK, Kim SW, Kim JS, et al. Bronchiolitis obliterans in the 1990s in Korea and the United States. Chest 2001;120:1101-6. [Crossref] [PubMed]
- Zhang L, Irion K, Kozakewich H, et al. Clinical course of postinfectious bronchiolitis obliterans. Pediatr Pulmonol 2000;29:341-50. [Crossref] [PubMed]
- Colom AJ, Teper AM, Vollmer WM, et al. Risk factors for the development of bronchiolitis obliterans in children with bronchiolitis. Thorax 2006;61:503-6. [Crossref] [PubMed]
- Koh YY, Jung DE, Koh JY, et al. Bronchoalveolar cellularity and interleukin-8 levels in measles bronchiolitis obliterans. Chest 2007;131:1454-60. [Crossref] [PubMed]
- Kurland G, Michelson P. Bronchiolitis obliterans in children. Pediatr Pulmonol 2005;39:193-208. [Crossref] [PubMed]
- Krumbholz A, Sandhaus T, Göhlert A, et al. Epstein-Barr virus-associated pneumonia and bronchiolitis obliterans syndrome in a lung transplant recipient. Med Microbiol Immunol 2010;199:317-22. [Crossref] [PubMed]
- Castro-Rodriguez JA, Daszenies C, Garcia M, et al. Adenovirus pneumonia in infants and factors for developing bronchiolitis obliterans: a 5-year follow-up. Pediatr Pulmonol 2006;41:947-53. [Crossref] [PubMed]
- Jensen SP, Lynch DA, Brown KK, et al. High-resolution CT features of severe asthma and bronchiolitis obliterans. Clin Radiol 2002;57:1078-85. [Crossref] [PubMed]
- Copley SJ, Padley SP. High-resolution CT of paediatric lung disease. Eur Radiol 2001;11:2564-75. [Crossref] [PubMed]
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015. at Available online: http://ginasthma.org/wp-content/uploads/2016/01/GINA_Report_2015_Aug11-1.pdf, accessed December 25, 2015.
- Chan PW, Muridan R, Debruyne JA. Bronchiolitis obliterans in children: clinical profile and diagnosis. Respirology 2000;5:369-75. [Crossref] [PubMed]
- Guilpain P, Mouthon L. Antiendothelial cells autoantibodies in vasculitis-associated systemic diseases. Clin Rev Allergy Immunol 2008;35:59-65. [Crossref] [PubMed]
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 1988;318:1651-7. [Crossref] [PubMed]
- Savige J, Dimech W, Fritzler M, et al. Addendum to the International Consensus Statement on testing and reporting of antineutrophil cytoplasmic antibodies. Quality control guidelines, comments, and recommendations for testing in other autoimmune diseases. Am J Clin Pathol 2003;120:312-8. [Crossref] [PubMed]
- Kobayashi S, Fujimoto S, Takahashi K, et al. Anti-neutrophil cytoplasmic antibody-associated vasculitis, large vessel vasculitis and Kawasaki disease in Japan. Kidney Blood Press Res 2010;33:442-55. [Crossref] [PubMed]
- Mahler M, Bogdanos DP, Pavlidis P, et al. PR3-ANCA: a promising biomarker for ulcerative colitis with extensive disease. Clin Chim Acta 2013;424:267-73. [Crossref] [PubMed]
- Yamada H. ANCA: associated lung fibrosis. Semin Respir Crit Care Med 2011;32:322-7. [Crossref] [PubMed]
- Ding YL, Zhu H, Yao WZ, et al. Clinical analysis of diffusive interstitial lung diseases with positive anti-neutrophil cytoplasmic antibody. Beijing Da Xue Xue Bao 2011;43:222-7. [PubMed]
- Chen DH, Lin YN, Lan SL, et al. Clinical characteristics of bronchiolitis obliterans in pediatric patients. Zhonghua Er Ke Za Zhi 2012;50:98-102. [PubMed]
- Wang W, Shen KL, Zeng JJ. Clinical studies of children with bronchiolitis obliterans. Zhonghua Er Ke Za Zhi 2008;46:732-8. [PubMed]
- Subspecialty Group of Respiratory Diseases, Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. Guidelines for management of childhood community acquired pneumonia (for trial implementation) (I). Zhonghua Er Ke Za Zhi 2007;45:83-90. [PubMed]
- Champs NS, Lasmar LM, Camargos PA, et al. Post-infectious bronchiolitis obliterans in children. J Pediatr (Rio J) 2011;87:187-98. [PubMed]
- Gosink BB, Friedman PJ, Liebow AA. Bronchiolitis obliterans. Roentgenologic-pathologic correlation. Am J Roentgenol Radium Ther Nucl Med 1973;117:816-32. [Crossref] [PubMed]
- Colom AJ, Teper AM. Clinical prediction rule to diagnose post-infectious bronchiolitis obliterans in children. Pediatr Pulmonol 2009;44:1065-9. [Crossref] [PubMed]
- Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int 1998;53:743-53. [Crossref] [PubMed]
- Xiao H, Heeringa P, Liu Z, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 2005;167:39-45. [Crossref] [PubMed]
- Jennette JC, Falk RJ, Hu P, et al. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol 2013;8:139-60. [Crossref] [PubMed]
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-53. [Crossref] [PubMed]
- Anderson RL, Hiemstra PS, Ward C, et al. Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Eur Respir J 2008;32:670-7. [Crossref] [PubMed]
- Fischer GB, Sarria EE, Mattiello R, et al. Post infectious bronchiolitis obliterans in children. Paediatr Respir Rev 2010;11:233-9. [Crossref] [PubMed]
- Liu XY, Jiang ZF, Peng Y. Clinical features of 23 cases of bronchilitis obliterans. Zhongguo Shi Yong Er Ke Za Zhi 2008;23:263-5.
- Sato M, Keshavjee S. Bronchiolitis obliterans syndrome: alloimmune-dependent and -independent injury with aberrant tissue remodeling. Semin Thorac Cardiovasc Surg 2008;20:173-82. [Crossref] [PubMed]
- Chiu CY, Wong KS, Huang YC, et al. Bronchiolitis obliterans in children: clinical presentation, therapy and long-term follow-up. J Paediatr Child Health 2008;44:129-33. [Crossref] [PubMed]
- Konstantinov KN, Ulff-Møller CJ, Tzamaloukas AH. Infections and antineutrophil cytoplasmic antibodies: triggering mechanisms. Autoimmun Rev 2015;14:201-3. [Crossref] [PubMed]
- Terrier B, Saadoun D, Sène D, et al. Antimyeloperoxidase antibodies are a useful marker of disease activity in antineutrophil cytoplasmic antibody-associated vasculitides. Ann Rheum Dis 2009;68:1564-71. [Crossref] [PubMed]
- Stegeman CA. Anti-neutrophil cytoplasmic antibody (ANCA) levels directed against proteinase-3 and myeloperoxidase are helpful in predicting disease relapse in ANCA-associated small-vessel vasculitis. Nephrol Dial Transplant 2002;17:2077-80. [Crossref] [PubMed]


