Complete response of myeloid sarcoma with cardiac involvement to radiotherapy
Introduction
Myeloid sarcoma (MS), also known as chloroma or granulocytic sarcoma, is a rare hematological phenomenon defined as the aggregation of immature myeloid cells outside the bone marrow. Most cases of MS have been diagnosed in acute myeloid leukemia (AML) patients, with a reported incidence of 2.5–9.1% (1). The incidence of isolated MS relapse after allogeneic hematopoietic stem cell transplantation (HSCT) varied in previous studies, ranging from less than 1% to 12% (2-4). Optimal treatments of MS remained uncertain. Chemotherapy was widely administrated upfront, whereas local radiotherapy was considered part of a combined modality treatment and performed the role of palliative symptoms relief, especially for rapidly progressive lesions (5,6).
Because MS can present at various extra-marrow lesion sites and result in prominent signs and symptoms, a high degree of awareness is mandatory for physicians caring for AML patients. Common sites of MS include the skin, head and neck, brain and spinal cord, and breasts (6). A cardiac site with MS involvement is extremely rare, and treatment results for cardiac MS have only been addressed by a few studies (7-14). Here, we report that an AML patient with isolated cardiac MS relapse after HSCT was successfully treated using fractionated radiotherapy and review studies on treatment modalities including chemotherapy and radiotherapy and treatment responses and outcomes of MS patients with cardiac involvement.
Case presentation
A 19-year-old man presented with AML, FAB (French-American-British) class M1, in June 2009. Cytogenetic studies revealed a normal karyotype, and a gene mutation screening was negative for nucleophosmin (NPM) and showed FMS-like tyrosine kinase 3. He received induction chemotherapy with idarubicin and cytarabine. However, left eye redness and pain developed after chemotherapy. A fundoscopy showed subretinal leukemic cell infiltration, but a cerebrospinal fluid examination was negative for leukemic cells. In addition, he received three cycles of intrathecal chemotherapy with methotrexate, cytarabine, and methylprednisolone and achieved complete remission (CR) in a subsequent bone marrow study. He completed consolidation chemotherapy with idarubicin and high-dose cytarabine without major complications. Matched-unrelated-donor peripheral blood stem cell transplantation was performed smoothly in January 2010.
He developed multiple, tiny lymphadenopathies in the region of left neck level II in September 2011. The magnetic resonance imaging of head and neck disclosed multiple tiny lymphadenopathies without obvious mass lesions. However, histopathologic examination of core needle biopsy of lymphadenopathies revealed chronic inflammation but negative for malignant cells. The patient subsequently received selective neck dissection of multiple tiny lymphadenopathies for confirming histologic diagnosis because he had AML-free for two years after initial treatment. The pathology of the lymphadenopathies showed MS, in which the tumor cells were positive for myeloperoxidase and CD117 and negative for CD34. He then received chemotherapy with mitoxantrone, etoposide, and intermediate-dose Ara-C and consolidative radiotherapy with 30 Gy in 15 fractions to the left neck level II tumor bed and 24 Gy in 15 fractions to the lymphatic regions of left levels III, IV, and V using a 6-MV photon, volumetric-modulated arc therapy (VMAT) technique. After the second CR, he received donor lymphocyte infusion in February 2012 but subsequently developed grade I–IIgrave-versus-host disease (GVHD) of the liver, skin, lungs, oral mucosa, and gastrointestinal tract. The GVHD signs and symptoms were controlled using cyclosporine and steroid. Subsequently, he also developed MS at the left preauricular skin area with parotid involvement. He underwent chemotherapy with FLAG (fludarabine + high-dose cytarabine + G-CSF) regimen followed by local radiotherapy with 30 Gy in 15 fractions to the left parotid gland and skin lesion and achieved CR again.
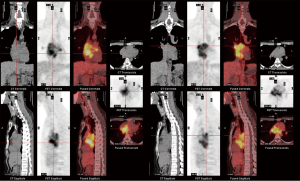
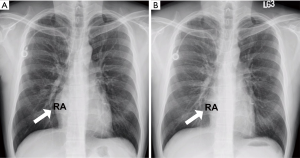
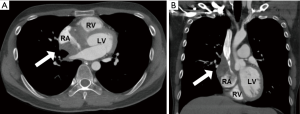
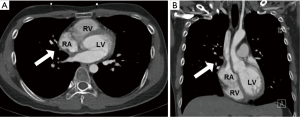
However, he developed sudden-onset chest tightness in November 2013, and a chest computed tomography (CT) scan disclosed a soft tissue mass along the interatrial groove with an encasement of the right upper pulmonary vein. The positron emission tomography scan revealed a large heterogeneous-intense hypermetabolic area involving the mediastinum and interarterial region (standard uptake value, maximum 8.3) (Figure S1). A percutaneous cardiac angiography with an intracardiac echocardiography-guided biopsy showed MS. Because of bronchiolitis obliterans caused by chronic GVHD and active pneumonia, radiotherapy was not suggested. After the pneumonia subsided, he received chemotherapy with azacitidine and achieved partial relief of the symptoms. However, in July 2014, he presented with superior vena cava (SVC) syndrome; a chest X-ray revealed right atrial enlargement (Figure S2A), and a cardiac CT scan revealed an enlarged right atrial tumor with venous encasement (Figure 1). Because of the SVC syndrome, he received radiotherapy with 24 Gy in 12 fractions. A forward “field in field” intensity-modulated radiation therapy (IMRT) technique with a 10-MV photon was used to treat his entire heart and involved the great vessels with 2 fields in an anterior-posterior direction. The radiotherapy plan is shown in Figure 2. The mean lung dose was 7.4 Gy, and the lung V20 (percentage of total lung volume receiving ≥20 Gy) was 19%. The maximum spinal cord dose was limited to less than 25 Gy. After the radiotherapy was completed, the SVC syndrome subsided. A follow-up CT 4 weeks after completing radiotherapy demonstrated CR of the MS cardiac tumor (Figure S2B, Figure 3). He remained MS-free without obvious radiation-related toxicities till the last follow up in January 2015. However, he died of sepsis-associated respiratory failure in February 2015.

Discussion
An optimal management of MS has not yet to be developed because of the rarity of this phenomenon and heterogeneous presentation. The treatment of choice depends on the status of the underlying disease and also on the MS lesion site. In general, MS emerging at presentation in combination with AML was treated as AML (5). The presence of MS was considered a poor prognostic factor for survival in some series (15). Nevertheless, the outcome of an MS relapse after transplantation seemed to be associated with a superior survival outcome (4,16). Chemotherapy is typically applied as a first-line treatment; however, there is no standard regimen for MS relapse. Donor lymphocyte infusion (17) and azacitidine (18) have been proposed as showing potential benefit on relapsed MS and AML. In our case, both donor lymphocyte infusion and azacitidine were used to achieve a short period free of progression.
Generally, radiotherapy serves as a palliation or consolidation treatment for MS, which we recently demonstrated in 20 patients receiving radiotherapy for MS lesions (in 43 radiotherapy courses) (6). The median radiotherapy dose of 20 Gy (range, 6–35 Gy), administered in 1.5–3.5 Gy fractions, provided a 63% CR rate, indicating that a modest RT dose (20–30 Gy) achieves strong local control of MS. Hall et al. also showed that approximately 50% of patients had subjectively complete symptomatic improvement without significant acute toxicities after palliative radiotherapy with a median dose of 24 Gy (19). However, when radiotherapy is used to treat cardiac involvement of MS, several concerns arise. The organ motion of the heart makes it difficult to define a tumor location precisely. Possible toxicity to the lungs, heart, and spinal cord should be considered because some of these patients have received HSCT or have impaired heart and lung function. Because modest radiotherapy doses of 20–30 Gy results in a favorable CR rate for MS, we treated our patient with 24 Gy in 12 fractions to the entire heart, using a forward “field in field” IMRT technique. The studied patient experienced a substantial improvement of symptoms during radiotherapy and had no acute lung and heart toxicity at presentation. In addition, we reviewed published case reports addressing cardiac MS and treatment outcomes (Table 1). Most cases received chemotherapy as a primary treatment and a possible HSCT if there was no history of previous transplantation. Radiotherapy was employed in two cases. One of them received 15 Gy in 10 fractions for cardiac MS followed by chemotherapy and an HSCT conditioning regimen with a total body irradiation of 12 Gy (8); the other received local radiotherapy to the heart using 24 Gy in 12 fractions, with a complete radiographic and clinical response and no acute toxicity being reported (10).
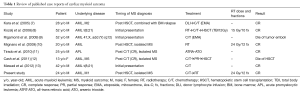
Full table
In our previously published data (6), patients who received radiotherapy after HSCT had a higher CR rate compared with those who underwent RT prior to HSCT (CR rate 100% vs. 50%, P=0.05). Radiation-induced graft-versus-leukemia effect may contribute to the favorable results of radiotherapy and may explain why our current case was alive 3 years after MS (20). In this study, we present a case of isolated intracardiac MS successfully treated by low-dose, fractionated radiotherapy. Based on our case and other case reports, cardiac involvement of MS can be treated safely with local radiotherapy with a favorable response. However, the addition of chemotherapy after radiotherapy for MS is warranted because of possible systemic failure and new MS merged outside the radiotherapy field following local treatment.
Acknowledgements
This study was supported by the following research grants: MOST 104-2314-B-002-189-MY3 from Ministry of Science and Technology, Taiwan, and NTUH 105-S3059 from National Taiwan University Hospital, Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Bakst R, Wolden S, Yahalom J. Radiation therapy for chloroma (granulocytic sarcoma). Int J Radiat Oncol Biol Phys 2012;82:1816-22. [Crossref] [PubMed]
- Békássy AN, Hermans J, Gorin NC, et al. Granulocytic sarcoma after allogeneic bone marrow transplantation: a retrospective European multicenter survey. Acute and Chronic Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 1996;17:801-8. [PubMed]
- Simpson DR, Nevill TJ, Shepherd JD, et al. High incidence of extramedullary relapse of AML after busulfan/cyclophosphamide conditioning and allogeneic stem cell transplantation. Bone Marrow Transplant 1998;22:259-64. [Crossref] [PubMed]
- Solh M, DeFor TE, Weisdorf DJ, et al. Extramedullary relapse of acute myelogenous leukemia after allogeneic hematopoietic stem cell transplantation: better prognosis than systemic relapse. Biol Blood Marrow Transplant 2012;18:106-12. [Crossref] [PubMed]
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood 2011;118:3785-93. [Crossref] [PubMed]
- Chen WY, Wang CW, Chang CH, et al. Clinicopathologic features and responses to radiotherapy of myeloid sarcoma. Radiat Oncol 2013;8:245. [Crossref] [PubMed]
- Kara IO, Sahin B, Paydas S, et al. Granulocytic sarcoma of the heart: extramedullary relapse of acute myeloblastic leukemia after allogeneic stem cell transplantation successfully treated by chemotherapy alone. Leuk Lymphoma 2005;46:1081-4. [Crossref] [PubMed]
- Kozelj M, Zorman D, Mrevlje B, et al. Cardiac granulocytic sarcoma diagnosed by intracardiac echocardiography-guided biopsy. Int J Hematol 2008;88:101-3. [Crossref] [PubMed]
- Rigamonti F, Beris P, Sanchez-Pareja A, et al. Atypical presentation of acute myeloid leukemia: cardiac myeloid sarcoma. Int J Hematol 2009;89:693-8. [Crossref] [PubMed]
- Mignano JE, Chan MD, Rosenwald IB, et al. Intracardiac chloroma. J Pediatr Hematol Oncol 2009;31:977-9. [Crossref] [PubMed]
- Tirado CA, Chen W, Valdez F, et al. Unusual presentation of myeloid sarcoma in a case of acute promyelocytic leukemia with a cryptic PML-RARA rearrangement involving multiple sites including the atrium. Cancer Genet Cytogenet 2010;200:47-53. [Crossref] [PubMed]
- Cash T, Becton D, Mian A. Cardiac myeloid sarcoma: a case report and review of literature. J Pediatr Hematol Oncol 2011;33:e330-2. [Crossref] [PubMed]
- Mawad R, Wu D, Abkowitz JL, et al. Myeloid sarcoma of the heart. Leuk Lymphoma 2012;53:2511-4. [Crossref] [PubMed]
- Liu PI, Ishimaru T, McGregor DH, et al. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer 1973;31:948-55. [Crossref] [PubMed]
- Breccia M, Mandelli F, Petti MC, et al. Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res 2004;28:1165-9. [Crossref] [PubMed]
- Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340-50. [Crossref] [PubMed]
- Wada A, Kobayashi N, Asanuma S, et al. Repeated donor lymphocyte infusions overcome a myeloid sarcoma of the stomach resulting from a relapse of acute myeloid leukemia after allogeneic cell transplantation in long-term survival of more than 10 years. Int J Hematol 2011;93:118-22. [Crossref] [PubMed]
- de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer 2010;116:5420-31. [Crossref] [PubMed]
- Hall MD, Chen YJ, Schultheiss TE, et al. Treatment outcomes for patients with chloroma receiving radiation therapy. J Med Imaging Radiat Oncol 2014;58:523-7. [PubMed]
- Chakraverty R, Flutter B, Fallah-Arani F, et al. The host environment regulates the function of CD8+ graft-versus-host-reactive effector cells. J Immunol 2008;181:6820-8. [Crossref] [PubMed]


