Severity of coronary artery disease and retinal microvascular signs in patients with diagnosed versus undiagnosed diabetes: cross-sectional study
Introduction
The incidence and prevalence of diabetes is rapidly increasing worldwide, and represents a significant social and economic burden to the healthcare system (1). In 2008, type 2 diabetes mellitus was the ninth leading cause of death worldwide (2). Diabetes is strongly associated with obesity, dyslipidemia, hypertension and chronic hyperglycemia. Diabetes also has multiple known sequelae including diabetic retinopathy, a leading cause of blindness in adults, and diabetic nephropathy, a leading cause of end-stage renal disease, as well as significant associations with strokes, cardiovascular events and diabetic neuropathy.
Several studies have demonstrated that individuals with undiagnosed or undetected diabetes are at higher risk of diabetic and cardiovascular complications. In the Second National Health and Nutritional Examination survey (3), approximately 50% of diabetes in all age groups was newly diagnosed during the study. A more recent report from a German population study (4) also demonstrated that out of 40% of the population aged 55–74 years in the region who had disturbed glucose tolerance or disease, approximately half of these individuals were undiagnosed prior to the study. Furthermore, it was demonstrated that in a higher risk cohort of patients undergoing cardiac bypass procedures, patients with undiagnosed diabetes had significantly higher morbidity and mortality compared to patients with diagnosed diabetes or no diabetes (5).
Data for outcomes of undiagnosed diabetes remains limited, and whether individuals with undiagnosed diabetes or prediabetic status have higher risks of cardiovascular, ophthalmological and neurological disease has not been well established. Diabetes also has major effects on the microvasculature and changes in retinal vessel calibres are linked to incident cardiovascular outcomes. Therefore, the present cross-sectional study was undertaken to assess coronary artery disease (CAD) extent and severity, and changes to the retinal microvascular structure in participants with undiagnosed versus diagnosed type 2 diabetes.
Methods
Study population
The AHES is a clinical cohort of 1,680 participants who were presented to a major tertiary referral hospital that services the greater western Sydney area (Westmead Hospital, Sydney, Australia) between June 2009 and January 2012 to evaluate potential CAD by coronary angiography. Biochemical, angiographic, clinical data, peripheral arteriolar wave form analysis, pulse wave velocity, ankle brachial pressure index, peripheral and invasive blood pressure measurements, echocardiography, electrocardiography, visual acuity and retinal photography data were collected on 1,680 participants. Ethics approval was obtained from the Western Sydney Local Health Network Human Research Ethics Committee. Informed consent was obtained from all patients for being included in the study.
All eligible patients presenting for assessment of suspected CAD were included in this study. Exclusion criteria were patients with history of coronary artery bypass graft or coronary artery stenting. These patients were excluded because the Gensini and Extent scoring systems used have not been well validated in these groups. Participants were also excluded if they had incomplete information on retinal microvasculature or absent Gensini or Extent scores.
Medical history
A 252-item questionnaire was used to obtain medical history, cardiovascular and familial risk factors. This included history of angina, myocardial infarction or angiography and intervention (coronary artery stent or coronary artery bypass graft, CABG), previous stroke, transient ischemic attack (TIA), hypertension, hypercholesterolemia, diabetes mellitus, current management of these chronic conditions, current medications, smoking status and alcohol consumption. Previous history of CHD (AMI, CABG) and/or coronary artery stent was determined by self-report and/or review of previous admissions.
Assessment of diabetes status
Participants with previously diagnosed diabetes were defined as those with a prior history of diabetes via the medical questionnaire described above, current use of hypo-glycemic agents, or those with a fasting plasma glucose (FPG) of ≥7.0 mmol/L. Participants with undiagnosed diabetes were defined as those who did not have a prior history of diabetes as per medical questionnaire, did not previously use hypoglycemic agents, but upon blood testing had an FPG ≥7.0 mmol/L.
Assessment of coronary artery disease (CAD)
Routine diagnostic coronary angiography was performed after 6 h of fasting via either the femoral or radial artery using a catheter of known dimensions (5–7 Fr). Selective coronary injections of Ultravist (Schering) were filmed in standard projections on a Siemens biplane radiographic unit (Siemens Healthcare, Germany).
All angiograms were analysed offline by a trained cardiologist masked to the results of the adjunctive investigations and retinal grading. The coronary artery segments were defined using the Syntax system, which divides the arterial tree into 16 segments based on the modified American Heart Association classification (6). For each segment, the severity of obstruction was documented using several grades: normal (0%), 1–25%, 25–50%, 50–74%, 75–99%, and 100% (occluded). Each lesion that was visually scored as greater than 50% luminal obstruction in a vessel that was ≥1.5 mm diameter was further analysed using quantitative coronary analysis (QCA). QCA was performed using validated computerised edge-detection software (QCA Plus, Sanders Data Systems, Palo Alto, California, USA).
Coronary angiograms were scored according to three methods to document the severity and extent of CAD:
- Vessel and segment score (severity score): vessel scores were calculated based on the number of vessels with significant obstructive CAD. The American College of Cardiology taskforce definition from 2011 uses 50% stenosis to define significant vessel disease (7). This definition was used for the left main coronary, right coronary, left anterior descending and left circumflex arteries. Scores ranged from 0 to 4, depending on the vessels with greater than 50% stenosis. Left main artery stenosis was scored as double vessel disease, as per coronary artery surgery study scoring system. The segment score was reported based on the number of obstructive lesions present in the 16 segments.
- Gensini score (severity score): this has been described previously. Briefly, the coronary arterial tree was divided into segments with multiplying factors according to the functional importance of any given segment (5 for the left main trunk to 0.5 for the most distal segments) and the percentage reduction in luminal diameter of each narrowing was assigned a score (0, 1, 2, 4, 8, 16 or 32), according to the degree of stenosis. The sum of the scores of all the segments gives the Gensini score, which places emphasis on the severity of the disease (8). Gensini scores were stratified into 4 groups. Group 1 has a Gensini score of 0, whilst groups 2–4 represents score tertiles.
- Extent score: the extent score was proposed by Sullivan et al. (9) to define the proportion of the coronary arterial tree with angiographically detectable coronary atheroma. The proportion of each vessel involved by the atheroma, identified by lumen irregularity, was multiplied by a factor for each vessel, which is related to the length of that vessel. The scores for each vessel were added to give a total score out of 100. This percentage represents the proportion of coronary intimal surface area containing coronary atheroma (8). Extent scores were stratified into 4 groups. Group 1 has an extent score of 0, whilst groups 2–4 represents score tertiles.
Retinal vascular caliber assessment
Participants had dilated, digital photographs taken of the optic disc and macula of both eyes using a Canon 60° fundus camera (Model CF-60DSi, Canon Inc., Tokyo, Japan) with an attached digital camera (Model 1DSmkIII, Canon Inc., Tokyo, Japan). Retinal vascular caliber measurements for the right eye of each participant were used. Left eye measurements were used if right eye photographs were un-gradable. One grader, masked to participant identity and characteristics, measured retinal vessel caliber using a computer-assisted program with high reproducibility. Details have been previously described (10,11). Based on a standard protocol, the grader measured the diameters of all arterioles and venules coursing through a specified area (0.5–1 disc diameter surrounding the optic disc) (12). Average retinal arteriolar and venular calibers were calculated using the Knudtson-Hubbard formula (12).
Statistical methods
SAS software (SAS Institute, Cary NC) v9.2 was used for analyses. Multivariable ordinal logistic regression models were used to assess the independent association between undiagnosed versus previously diagnosed diabetes with Gensini scores, extent scores, and retinal arteriolar and venular caliber. Regression analysis was first adjusted for age and sex, and additional confounders that were likely to influence observed associations based on findings from published literature were also adjusted for. Potential confounders that were assessed included: ethnicity, BMI, smoking, BP, cardiac biomarkers (e.g., serum triglycerides, cholesterol, creatinine kinase, cardiac troponin T and high sensitivity cardiac troponin T), and various medical conditions (e.g., hypertension). Only those potential confounders that significantly modified the effect of diabetes status on our outcome of interest (by ≥10%) or predicted key study outcomes (e.g., presence of stenotic lesions, Gensini and extent scores, and retinal vessel caliber) in age-sex adjusted models were included in subsequent multivariable analyses. Thus, final, parsimonious models were adjusted for age, sex, and BMI, hypertension, smoking status, and eGFR; as these covariates satisfied the above criteria for inclusion. Estimated odds ratios (OR) and 95% confidence intervals (CIs) are presented for associations between diabetes status (undiagnosed vs. previously diagnosed) and coronary angiographic scores (Gensini, extent scores) or the retinal microvasculature (retinal arteriolar and venular diameters).
Results
Subject characteristics
From the 1,680 participants of the AHES, there were 76 participants (4.5%) who had undiagnosed diabetes compared to 489 participants (29.1%) with prior known diagnosis of diabetes. The prevalence of undiagnosed diabetes in the AHES is similar to prevalence rates reported in several other population studies (13-15).
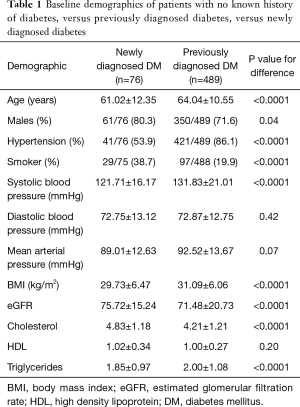
Table 1 shows the study characteristics of participants with undiagnosed diabetes and with previously diagnosed diabetes. Participants with undiagnosed diabetes were more likely to be males and smokers, but less likely to have hypertension compared to participants with diagnosed diabetes. Systolic BP was significantly lower in the undiagnosed group. Undiagnosed participants versus participants with diagnosed diabetes had significantly lower BMI and higher eGFR. Cholesterol levels were lower but triglyceride levels were higher in undiagnosed compared to previously diagnosed participants.
Full table
Relationship between diabetes status and CAD extent and severity
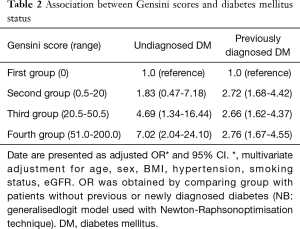
Table 2 shows a significant association between status of diagnosed/undiagnosed diabetes and severity of CAD, as measured using the Gensini score. Patients with undiagnosed diabetes compared to those with no diabetes had 7.02-fold increase odds of being in the highest quartile of Gensini scores (multivariate adjusted OR =7.02; 95% CI, 2.04–24.1). A weaker but still significant association was seen for previously diagnosed diabetes and Gensini score (i.e., highest versus lowest quartile), with multivariate adjusted OR =2.76 (95% CI, 1.67–4.55).
Full table
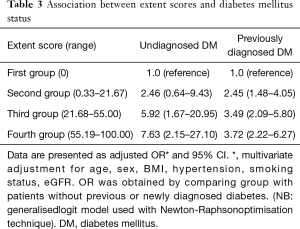
Association between diabetes status and extent of CAD is demonstrated in Table 3. Again, patients with undiagnosed diabetes had a 7.6-fold increase in odds of having Extent scores of the highest quartile (multivariate adjusted OR =7.63; 95% CI, 2.15–27.10). Participants with previously diagnosed diabetes had a 3.7-fold increase in odds of having Extent scores of the highest quartile (multivariate adjusted OR =3.72; 95% CI, 2.22–6.27).
Full table
Relationship between diabetes status and retinal vascular caliber
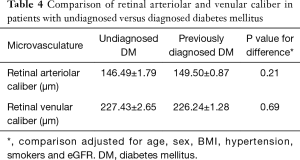
Table 4 demonstrates no significant difference between participants with undiagnosed diabetes and previously diagnosed diabetes in terms of changes to the retinal arteriolar diameter (P=0.21) and retinal venular diameter (P=0.69), following multivariable adjustment for age, sex, BMI, hypertension, smoker status and eGFR.
Full table
Discussion
This epidemiological study is the first to show that participants with undiagnosed diabetes had a strong and independent association with both the extent and severity of CAD, through the use of coronary angiography in a symptomatic population. This observed association was less marked in those with previously diagnosed diabetes. No significant differences in adjusted mean retinal arteriolar or venular diameters were observed between participants with both diagnosed and undiagnosed diabetes.
In one of the earliest, extensive population-based studies on cardiovascular disease, the Framingham study in 1974 demonstrated a significant 2–3-fold increase in cardiovascular sequelae in persons with diabetes versus non-diabetes, after adjustment for other risk factors such as age, gender, BMI, hypercholesterolemia and hypertension (16,17). This association was also observed in patients with preclinical diabetes or impaired fasting glucose. In a prospective cohort study of 11,554 white men and 666 black men, the Chicago Heart Association (CHA) Detection Project in Industry demonstrated increased mortality risk in both preclinical and clinical diabetes groups (18). Despite increasing efforts in risk factor screening and preventative measures for diabetes worldwide, the prevalence of undiagnosed diabetes continues to pose a major health problem. This is a pertinent issue, given that sequelae including retinopathy, micro-vascular and macro-vascular complications are developing during the period of non-detection. Indeed, the prevalence of macrovascular disease in undiagnosed diabetes has been suggested to be approximately equal to that of diagnosed diabetes, both being at least twice that for patients without diabetes (3). The Second National Health and Nutrition Examination Survey data (3) suggests that approximately 50% of participants with diabetes in all age groups are previously undiagnosed, and this trend has been further corroborated by several other population studies (18-21). In higher risk populations such as patients undergoing cardiac bypass surgery, the prevalence of undiagnosed diabetes was found to be 5.2%, compared to 29.6% of participants with diagnosed diabetes, which is similar to estimates from our study (5). A study of 7,310 bypass patients (5) demonstrated significantly higher resuscitation and reintubation rates in the undiagnosed group, and also the highest perioperative mortality rate. However, the evidence for outcomes of undiagnosed diabetes remains limited, no study to date has compared the severity and extent of cardiovascular disease and retinal microvasculature between participants with undiagnosed versus previously diagnosed diabetes.
These questions were addressed by analysis of our study, the Australian Heart Eye Study (AHES), a clinic-based cross-sectional study of 1,680 individuals who presented for evaluation of suspected CAD, who also had retinal examination performed (22-24). In this cohort, the prevalence of undiagnosed diabetes was 4.5% and previously diagnosed diabetes was 29.1%. The prevalence of undiagnosed diabetes is similar to values reported in a higher risk cardiac bypass population by Lauruschkat et al. (5) (5.2%). The Bangladesh Population-based Diabetes and Eye Study (25) recently reported a 3.6% prevalence of undiagnosed diabetes, based on a random sampling strategy. Prevalence rates in the general population appear to be lower, with reported 1.13% undiagnosed type 2 diabetes in the Canadian Health Measures Survey (26), 2.7% in the Third National Health and Nutrition Examination Survey in the U.S. (19), and 2.6% prevalence in rural communities in Sudan (27).
The AHES determined Gensini and extent scores, as indicators of the severity and extent of CAD. There is a paucity of studies in the literature, which have used these methods to compare extent and severity of CAD in participants with previously diagnosed versus undiagnosed diabetes. In the present study, participants with undiagnosed diabetes were 7-fold more likely to have the worse (highest) Gensini scores, while a 2.76-fold increase in odds was seen in participants with previously diagnosed diabetes. As such, based on the Gensini system, the severity of CAD appears to be worse in patients with diabetes, particularly in undiagnosed patients, compared to non-diabetic individuals. Similar associations were also observed using the extent score, which is considered a measure of the burden of coronary atheroma, regardless of severity of stenosis (9). Participants with undiagnosed compared to previously diagnosed diabetes had greater odds of having the highest Extent scores (i.e., worse CAD). Hence, the AHES provides epidemiological evidence of CAD that is more severe and extensive in the participants with undiagnosed versus previously diagnosed diabetes. The diagnosed group is more likely to have rigorous risk factor control measures, medical therapy and interventions in place which could reduce the odds of having severe and extensive CAD, compared to the undiagnosed cohort. As such, the above results demonstrate that whilst diabetes continues to be a global health issue, this situation is further exacerbated by undiagnosed diabetes and emphasises the need for improved community and clinical screening programs, education and preventative measures.
No differences in retinal microvasculature was observed between undiagnosed versus previously diagnosed cohorts, which is somewhat unsurprising, given that changes to the retinal microvascular structure are generally considered to be early biomarkers of systemic microvascular damage (28-30). It is likely that the present study did not have adequate statistical power to detect any modest associations between diabetes status and retinal vessel caliber changes.
Strengths of the present study include the novel measures of CAD severity through Gensini and extent scores, standardised measurements via coronary angiography, as well as standardised measurements for retinal vessel caliber. The present study is limited by several constraints. Firstly, the cross-sectional design of the AHES cohort with lack of follow-up data prevents inference of any causal relationships. Another limitation was that baseline characteristics and comorbidities were self-reported using a medical questionnaire. Residual confounding from unmeasured or unknown factors such as lifestyle or behavioral factors cannot be excluded as well. This data may be subjected to recall bias, which may undermine the validity of the data presented. Additionally, the AHES is a clinical cohort of patients who presented for suspected CAD. As such, the results may not be applicable for the general community population.
In summary, the present study demonstrated that more severe and extensive CAD was observed in participants with undiagnosed diabetes compared to those with previously diagnosed diabetes. There was no independent association detected between diabetes status and retinal microvascular signs. These results suggest the need for more rigorous screening and detection of diabetes in the clinical and community population, to prevent potential cardiovascular complications.
Acknowledgements
Funding: National Health and Medical Research Council (NHMRC) Project Grant 571012.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval was obtained from the Western Sydney Local Health Network Human Research Ethics Committee. Informed consent was obtained from all patients for being included in the study.
References
- Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:2568-9; author reply 2569. [Crossref] [PubMed]
- WHO. Global Status Report on noncommunicable diseases 2010. Available online: http://www.who.int/nmh/publications/ncd_report2010/en/
- Harris MI. Undiagnosed NIDDM: clinical and public health issues. Diabetes Care 1993;16:642-52. [Crossref] [PubMed]
- Rathmann W, Haastert B, Icks A, et al. High prevalence of undiagnosed diabetes mellitus in Southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia 2003;46:182-9. [PubMed]
- Lauruschkat AH, Arnrich B, Albert AA, et al. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation 2005;112:2397-402. [Crossref] [PubMed]
- Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219-27. [PubMed]
- Weintraub WS, Karlsberg RP, Tcheng JE, et al. ACCF/AHA 2011 key data elements and definitions of a base cardiovascular vocabulary for electronic health records: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards. J Am Coll Cardiol 2011;58:202-22. [Crossref] [PubMed]
- Norgaz T, Hobikoglu G, Aksu H, et al. Retinopathy is related to the angiographically detected severity and extent of coronary artery disease in patients with type 2 diabetes mellitus. Int Heart J 2005;46:639-46. [Crossref] [PubMed]
- Sullivan DR, Marwick TH, Freedman SB. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J 1990;119:1262-7. [Crossref] [PubMed]
- Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999;106:2269-80. [Crossref] [PubMed]
- Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 2002;287:1153-9. [Crossref] [PubMed]
- Knudtson MD, Lee KE, Hubbard LD, et al. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 2003;27:143-9. [Crossref] [PubMed]
- Xu W, Xu Z, Jia J, et al. Detection of prediabetes and undiagnosed type 2 diabetes: a large population-based study. Canadian Journal of Diabetes 2012;36:108-13. [Crossref]
- Leong A, Dasgupta K, Chiasson JL, et al. Estimating the population prevalence of diagnosed and undiagnosed diabetes. Diabetes Care 2013;36:3002-8. [Crossref] [PubMed]
- Bener A, Zirie M, Janahi IM, et al. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract 2009;84:99-106. [Crossref] [PubMed]
- Garcia MJ, McNamara PM, Gordon T, et al. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes 1974;23:105-11. [Crossref] [PubMed]
- Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation 1979;59:8-13. [Crossref] [PubMed]
- Lowe LP, Liu K, Greenland P, et al. Diabetes, asymptomatic hyperglycemia, and 22-year mortality in black and white men. The Chicago Heart Association Detection Project in Industry Study. Diabetes Care 1997;20:163-9. [Crossref] [PubMed]
- Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care 1998;21:518-24. [Crossref] [PubMed]
- Meigs JB, Nathan DM, Wilson PW, et al. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance. The Framingham Offspring Study. Ann Intern Med 1998;128:524-33. [Crossref] [PubMed]
- Saydah SH, Loria CM, Eberhardt MS, et al. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 2001;24:447-53. [Crossref] [PubMed]
- Wang SB, Mitchell P, Plant AJ, et al. Prevalence and risk factors of epiretinal membrane in a cohort with cardiovascular disease risk, compared with the Blue Mountains Eye Study. Br J Ophthalmol 2015;99:1601-5. [Crossref] [PubMed]
- Phan K, Mitchell P, Liew G, et al. Relationship between macular degeneration with prevalent heart failure: a cross-sectional population study. Int J Cardiol 2015;182:213-5. [Crossref] [PubMed]
- Phan K, Mitchell P, Liew G, et al. Relationship between macular and retinal diseases with prevalent atrial fibrillation - an analysis of the Australian Heart Eye Study. Int J Cardiol 2015;178:96-8. [Crossref] [PubMed]
- Islam FM, Chakrabarti R, Islam MT, et al. Prediabetes, diagnosed and undiagnosed diabetes, their risk factors and association with knowledge of diabetes in rural Bangladesh: The Bangladesh Population-based Diabetes and Eye Study. J Diabetes 2016;8:260-8. [Crossref] [PubMed]
- Rosella LC, Lebenbaum M, Fitzpatrick T, et al. Prevalence of prediabetes and undiagnosed diabetes in Canada (2007-2011) according to fasting plasma glucose and HbA1c screening criteria. Diabetes Care 2015;38:1299-305. [Crossref] [PubMed]
- Noor SK, Bushara SO, Sulaiman AA, et al. Undiagnosed diabetes mellitus in rural communities in Sudan: prevalence and risk factors. East Mediterr Health J 2015;21:164-70. [PubMed]
- Tabatabaee A, Asharin MR, Dehghan MH, et al. Retinal vessel abnormalities predict coronary artery diseases. Perfusion 2013;28:232-7. [Crossref] [PubMed]
- Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J 2007;28:1984-92. [Crossref] [PubMed]
- Tedeschi-Reiner E, Strozzi M, Skoric B, et al. Relation of atherosclerotic changes in retinal arteries to the extent of coronary artery disease. Am J Cardiol 2005;96:1107-9. [Crossref] [PubMed]


