Exophiala pisciphila: a novel cause of allergic bronchopulmonary mycosis
Introduction
Allergic bronchopulmonary mycosis (ABPM) is a hypersensitivity response to fungal antigens. The most common of these is Aspergillus fumigatus, in which case the condition is termed allergic bronchopulmonary aspergillosis (ABPA). It is estimated that 1% to 2% of patients with asthma will develop ABPA (1-3). However, other species and fungi can elicit the response. The clinical presentation usually consists of severe asthma, often refractory to treatment, short of systemic corticosteroids.
Exophiala pisciphila is one of 28 species of the genus Exophiala, a marine dematiaceous (dark in color) fungus belonging to the ‘black yeasts’ family. It was described by Carmichael in 1966 (4). Since then, it has been implicated as a cause of systemic mycosis in several species of fish. The only human infection was a case of skin abscess in an immunocompromised liver transplant patient reported in 1991 (5). It was recently isolated as a microbial contaminant of dialysis water in six hemodialysis units in Brazil (6). We herein report this organism as a cause of ABPM in a patient with severe persistent asthma.
Case presentation
A 67-year-old gentleman with adult-onset asthma had now become steroid-dependent. His severe-persistent asthma required eight admissions to another facility during the prior three years, each coinciding with the end of a prednisone taper. He was currently being seen for the first time in the pulmonary clinic following the most recent discharge. His baseline functional status was an ability to walk about 20 meters, after which dyspnea would become incapacitating. He had a daily cough with wheezing, and often expectorated brown-to-greenish thick sputum and plug-shaped mucus. This was associated with anterior rhinitis, but no fever, chills or chest pain. He required oxygen intermittently. His treatment regimen included budesonide/formoterol 160–4.5 twice daily, ipratropium, montelukast and prednisone 20 mg daily. He required rescue albuterol several times a day.
He had smoked a few cigarettes daily several years ago and had been exposed to liquid steel for more than 30 years while working at a steel mill. His father had asthma. A main hobby of his was fishing and frequenting fisher’s markets to purchase fresh catch.
Physical exam revealed a well-nourished male who was coughing intermittently. His chest exam showed limited excursion with decreased breath sounds throughout all fields, and mild pan-respiratory wheezing. No clubbing or cyanosis was noted.
Results
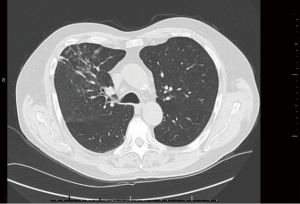
Chest imaging revealed right upper lobe infiltrates which subsided on future imaging (Figure 1). A review of prior chest X-rays was notable for fleeting infiltrates. Pulmonary function testing revealed mild obstruction with only partial bronchodilator response. Radioallergosorbent testing uncovered allergies to the molds Aspergillus fumigatus (1.2 kU/L; class II) and Cladosporium herbarum (1.08 kU/L; class II), as well as ragweed (0.16 kU/L; class 0/I) and elm (0.23 kU/L; class 0/I). Aspergillus antigen was however negative. IgE level was 2,036 international units per milliliter (IU/mL) with otherwise normal immunoglobulin levels. Records from a year earlier noted a blood eosinophil count of 2,350 cells/μL. The level had normalized after completing a prednisone course. It later increased to above 1,000 cells/μL during a recent exacerbation. Autoimmune workup with ANA, ENA and rheumatoid factor was unrevealing.
Following our encounter, sputum testing obtained at the end of his current course of prednisone revealed no eosinophils; gram stain noted black yeast, and the culture grew Exophiala pisciphila, which was further identified based on phenotypic characteristics, sequencing of the ITS region and BLAST search. Antifungal susceptibility testing was performed at 30 °C after 5 days using Sensititre YeastOne kit, and revealed the organism to be sensitive to itraconazole (Table 1). To ensure this organism was not a contaminant, sputum cultures were repeated and the same yeast was re-identified. Transbronchial biopsies however revealed no invasive elements or other interstitial disease.
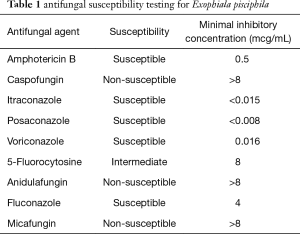
Full table
In the meantime, two doses of omalizumab were administered by an allergist, after which the patient remained very symptomatic and corticosteroid-dependent. No more doses were given due to logistical reasons prohibiting timely and effective dosing titration. At that point, the second set of cultures had been finalized and the decision was made to initiate therapy with itraconazole at 200 mg twice daily for 16 weeks. Within a week, he reported significant decrease in the use of albuterol and prednisone tapering was initiated. The latter was eventually discontinued 6 weeks later, at which point he reported near normal functional capacity and resolution of all respiratory symptoms. Pulmonary function testing revealed no worsening of airflow obstruction. Therapy with omalizumab eventually resumed on a 4-weekly basis, in order to ensure remission after completion of antifungal therapy.
Regular follow-ups for a year following the initiation of therapy noted stable pulmonary status with no symptoms. His asthma did not require prednisone use again. He continued to receive the same bronchodilator regimen, with occasional use of albuterol for rescue. There was no blood eosinophilia and the IgE level decreased to 228 IU/mL. Surveillance sputum cultures were negative for any fungus.
Discussion
ABPM is typically marked by uncontrolled asthma, often associated with fatigue, cough, mucus plugs, fever and weight loss. Hemoptysis has been rarely reported. Infiltrates on chest X-ray may be fleeting, and serial images may show them to be transient or permanent (7). With time, inflammation may lead to scarring, hence the fixed finding. On the other hand, high resolution chest tomography is the radiographic test of choice to demonstrate mucus impaction, peri-bronchial thickening, and central bronchiectasis—a hallmark of ABPM (8).
Laboratory testing reveals peripheral eosinophilia and elevated IgE levels (usually above 1,000 IU/mL). Following diagnosis, it is recommended to monitor IgE levels every 4–8 weeks for at least 1 year (9). Expectorated sputum and bronchoalveolar samples may reveal an elevated eosinophil count as well. The fungal elements may be retrieved from the airways by sputum culture or bronchoscopy, though this is not diagnostic in and of itself. However, tissue invasion is not observed. Reversibility on pulmonary function testing with inhaled bronchodilators may be elicited less than in asthmatics without ABPA.
To date, no comprehensive diagnostic criteria have been firmly established for ABPM. The most commonly accepted diagnostic criteria for ABPA (and by extension ABPM) were proposed by Rosenberg et al. in 1977 (10) and Patterson et al. in 1986 (11). Major criteria: asthma, type I cutaneous hypersensitivity reaction to antigens of the etiologic fungus (not all antigens are commercially available, including Exophiala), total IgE >1,000 ng/mL (416 IU/mL), serum fungus-specific IgE/IgG, serum precipitins to the etiologic fungus, fleeting pulmonary opacities, central bronchiectasis, and eosinophilia. Minor criteria: expectoration of mucus plugs, delayed/type III reaction, culture of fungus.
Stevens et al. proposed a simplified version in 2003 (12), excluding eosinophilia and precipitating antibodies, which may be negative when the patient is receiving corticosteroids, or during remission. Nonetheless, controversy regarding such criteria remains, as reflected by a 2012 survey carried out by the American Academy of Allergy, Asthma and Immunology (13). Several ABPM cases have been reported in non-asthmatics (14,15), even though asthma and cystic fibrosis remain the most important underlying conditions. Furthermore, ABPM is considered to be associated with atopy; hence rhinosinusitis, allergic conjunctivitis or atopic dermatitis may coexist.
In a 2012 review, Knutsen et al. presented the following diagnostic criteria: asthma or cystic fibrosis with deterioration of lung function, immediate Aspergillus species skin test reactivity, total serum IgE level greater than 416 IU/mL, increased Aspergillus-specific IgE and IgG antibodies, and chest radiographic infiltrates. Additional criteria might include peripheral blood eosinophilia, Aspergillus species serum precipitating antibodies, central bronchiectasis, and Aspergillus species-containing mucus plugs (16).
Systemic steroids are the mainstay of treatment, with the goal of decreasing IgE levels by up to 50% within 6–8 months (9). However, they may not prevent exacerbations or decline in spirometry. Thus appropriate antifungal therapy may be required. A Cochrane systematic review revealed that treatment with itraconazole for 16 weeks in ABPA permitted a reduction in corticosteroid dosage, improved clinical outcomes and led to a reduction in both IgE levels and sputum eosinophil count (17). Successful omalizumab use has also been reported in patients with ABPA (18,19). Such experience is lacking in the treatment of ABPM.
Conclusions
ABPM has surfaced as an important pulmonary disease in atopic patients. While Aspergillus remains the most common cause of ABPM, there is a plethora of other organisms which may precipitate this disease, thus complicating management and control of asthma. A global review of ABPM did not report Exophiala as a known culprit (15). In this review, the commonest etiologic agent was Candida albicans, reported in 60% of the cases, followed by Bipolaris species (13%), Schizophyllum commune (11%), Curvularia species (8%), Pseudallescheria boydii species complex (3%) and rarely, Alternaria alternata, Fusarium vasinfectum, Penicillium species, Cladosporium cladosporioides, Stemphylium languinosum, Rhizopus oryzae, Candida glabrata, Saccharomyces cerevisiae and Trichosporon beigelii. Most cases were reported in India (47%) followed by Japan. The median IgE value was threefold higher than that of ABPA, indicating that these fungi may elicit a stronger immunological response than Aspergillus (20).
In this report, we invite attention unto a marine organism that has thus far not been reported as a cause of ABPM. In our patient, four major and two minor diagnostic criteria were fulfilled, and other features were present (e.g., lack of tissue invasion and no response to inhaled bronchodilator). Successful treatment and eradication of Exophiala pisciphila led to complete symptom control of severe asthma, discontinuation of systemic steroids, and improvement in serum IgE levels and eosinophilia. While both omalizumab and itraconazole were administered, the highest benefit coincided with the latter therapy. We thus believe that proper awareness, testing and treatment of respiratory mycoses if identified, and the development of directed antigenic testing and precipitating antibodies for various fungi can be very helpful in managing difficult-to-control asthmatics.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lazarus AA, Thilagar B, McKay SA. Allergic bronchopulmonary aspergillosis. Dis Mon 2008;54:547-64. [Crossref] [PubMed]
- Agarwal R. Allergic bronchopulmonary aspergillosis. Chest 2009;135:805-26. [Crossref] [PubMed]
- Patterson K, Strek ME. Allergic bronchopulmonary aspergillosis. Proc Am Thorac Soc 2010;7:237-44. [Crossref] [PubMed]
- Carmichael JW. Cerebral mycetoma of trout due to a phialophora-like fungus. Sabouraudia 1966;5:120-3. [Crossref] [PubMed]
- Sughayer M, DeGirolami PC, Khettry U, et al. Human infection caused by Exophiala pisciphila: case report and review. Rev Infect Dis 1991;13:379-82. [Crossref] [PubMed]
- Figel IC, Marangoni PR, Tralamazza SM, et al. Black yeasts-like fungi isolated from dialysis water in hemodialysis units. Mycopathologia 2013;175:413-20. [Crossref] [PubMed]
- Shah A. Radiological aspects of allergic bronchopulmonary aspergillosis and allergic Aspergillus sinusitis: pathology. In: Kurup VP, editor. Mold allergy, biology and pathogenesis. Trivandrum, India: Research Signpost, 2005:163-74.
- Angus RM, Davies ML, Cowan MD, et al. Computed tomographic scanning of the lung in patients with allergic bronchopulmonary aspergillosis and in asthmatic patients with a positive skin test to Aspergillus fumigatus. Thorax 1994;49:586-9. [Crossref] [PubMed]
- Chupp G, Rochester GL. Allergic bronchopulmonary aspergillosis (mycosis). In: Fishman AP, Elias JA, Fishman JA, et al., editors. Fishman’s pulmonary diseases and disorders, 4th ed. New York, USA: Tata McGraw-Hill, 2008;837-44.
- Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977;86:405-14. [Crossref] [PubMed]
- Patterson R, Greenberger PA, Halwig JM, et al. Allergic bronchopulmonary aspergillosis. Natural history and classification of early disease by serologic and roentgenographic studies. Arch Intern Med 1986;146:916-8. [Crossref] [PubMed]
- Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003;37 Suppl 3:S225-64. [Crossref] [PubMed]
- Greenberger PA, Bush RK, Demain JG, et al. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014;2:703-8. [Crossref] [PubMed]
- Gendrya T, Moreaua S, Pelageb JP, et al. Allergic bronchopulmonary candidiasis in a marijuana smoker. Respir Med Extra 2006;2:27-9. [Crossref]
- Ishiguro T, Takayanagi N, Tokunaga D, et al. Pulmonary Schizophyllum commune infection developing mucoid impaction of the bronchi. Yale J Biol Med 2007;80:105-11. [PubMed]
- Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 2012;129:280-91. [Crossref] [PubMed]
- Wark PA, Gibson PG, Wilson AJ. Azoles for allergic bronchopulmonary aspergillosis associated with asthma. Cochrane Database Syst Rev 2004.CD001108. [PubMed]
- Tillie-Leblond I, Germaud P, Leroyer C, et al. Allergic bronchopulmonary aspergillosis and omalizumab. Allergy 2011;66:1254-6. [Crossref] [PubMed]
- Moss RB. The use of biological agents for the treatment of fungal asthma and allergic bronchopulmonary aspergillosis. Ann N Y Acad Sci 2012;1272:49-57. [Crossref] [PubMed]
- Chowdhary A, Agarwal K, Kathuria S, et al. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol 2014;40:30-48. [Crossref] [PubMed]


