Shedding light on the gray zone
Over two decades have passed since Pijls et al. proposed that invasively-determined myocardial fractional flow reserve (FFR) could serve as an index of the functional severity of coronary artery stenosis (1). FFR is derived from the ratio of mean pressure at the distal epicardial coronary conduit of an atheromatous coronary lesion to that of the aortic root in pharmacologically induced hyperemia, and represents the very fraction of maximal myocardial blood flow (MBF) that can be maintained despite coronary artery stenosis. The theoretical normal value of FFR is 1.00 regardless of the patient, the specific vessel studied or concurrent hemodynamic changes. However, when the microcirculation remains intact, the FFR value falls with the progression of a flow-limiting coronary lesion and as an increasing amount of myocardium becomes supplied by a flow-limiting coronary artery. Sequential non-invasive exercise tests, myocardial perfusion imaging (MPI) with single-photon emission computed tomography (SPECT) and stress echocardiography have confirmed that an FFR value of ≤0.75 represents stress-inducible myocardial ischemia (2). The DEFER trial (3) demonstrated that the revascularization (REV) of coronary stenosis with FFR >0.75 did not improve clinical outcomes compared with those of patients deferred to receive optimal medical treatment (OMT). Otherwise, the subsequent FAME II (Fractional Flow Reserve Guided PCI versus Medical Therapy in Stable Coronary Disease II) clinical trial (4) showed that REV of coronary stenosis with FFR ≤0.80 improved clinical outcomes compared with OMT alone. Thus, FFR values between 0.75 and 0.80 are referred to as the FFR gray zone, namely, an area of uncertainty regarding the actual degree of ischemia in patients with stable coronary artery disease (CAD). This gray zone of uncertainty affects decisions about which patients are selected for REV and those that will receive OMT.
The authors focused on patients in the gray zone, and confirmed the prognostic legitimacy of REV in such patients. They classified 1,459 patients with single-segment disease and FFR values within three strata as ischemic, gray zone and non-ischemic (0.70–0.75, 0.76–0.80 and 0.81–0.85, respectively) in a retrospective single-center study. The clinical endpoints of major adverse cardiac events (MACE) defined as the composite of overall death, myocardial infarction (MI) and target vessel REV were assessed in 1,010 of the patients who received OMT alone and 449 who were treated by REV + OMT and were followed up for 25 (range, 6–48) and 26 (range, 13–47) months, respectively. Although differences in MACE rates between patients treated with OMT alone and with REV + OMT were not statistically significant in the gray zone, trends towards higher rates of death or MI and overall death were observed in the group treated with OMT alone in comparison with REV + OMT (9.4% vs. 4.8%, P=0.06 and 7.5% vs. 3.2%, P=0.059, respectively). An increase in the MACE rate was statistically significant across the three FFR strata in the OMT group, especially when the lesion was proximally located. Otherwise, the MACE rate remained similar in the REV + OMT group regardless of the actual FFR value. These findings could serve from a prognostic viewpoint as a rationale for selecting REV to treat patients in the gray zone especially those with proximal lesions.
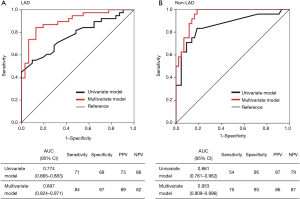
This evidence has also raised the issue of how to non-invasively diagnose patients who have stable CAD and flow-limiting coronary lesions corresponding to FFR ≤0.80. Coronary computed tomographic angiography (CCTA) derived FFR (FFRCT) is a novel and promising non-invasive approach that can precisely localize flow-limiting coronary artery stenosis as it applies computational fluid dynamics to calculate the FFR of each of three vessels from image acquisition by the standard rest CCTA study without a need for vasodilator-stress conditions. Although its application to severely calcified coronary arteries and patients with chronic kidney disease is somewhat limited, FFRCT might serve as a promising gatekeeper for invasive FFR assessment in routine clinical practice, because clinical data have shown strong correlation with invasive FFR and a reduction in false-positive findings in standard interpretation of CCTA images (5). Another potent modality that could address this issue is MPI-SPECT, because a nuclear sub-study of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial (6) demonstrated that MPI-SPECT findings could predict improved outcomes after REV. However, to predict FFR solely by standard interpretation of MPI-SPECT images seems somewhat limited, because MPI-SPECT findings represent changes in relative MBF between hyperemia and the resting state that can be affected by microcirculatory and myocardial properties in addition to epicardial coronary perfusion. Furthermore, interpretation is dependent on the presence and accurate identification of a region of normal perfusion. This is a particular impediment in diffuse or multi-vessel disease that could include, “balanced ischemia” and a scant obviously normal reference region (7). Actually, FFR and invasive coronary flow reserve (CFR) values have occasionally been mismatched, especially in patients with diffuse diseases of epicardial conduit vessels or diseases of the coronary microvasculature (8). We recently showed that a flow-limiting FFR of <0.80 could be predicted from findings of quantitative MPI with quantitative perfusion SPECT (QPS) and other non-invasive parameters identified by multivariate analyses (Table 1) (9) by assessing 136 diseased vessels in prospectively-identified 84 patients with stable CAD who were assessed by MPI-SPECT and invasive FFR. The formulas based on these analyses demonstrated to predict major vessels of interest with FFR <0.80 with defined probabilities (sensitivity, specificity and accuracy for LAD and non-LAD: 84%, 87% and 86%, and 75%, 93% and 87%, respectively) (Figure 1) (9). Although somewhat limited by a sample size and a single-center design, an appropriately-designed validation cohort study might provide a novel adjunctive tool that could diagnose functionally significant CAD from MPI findings.
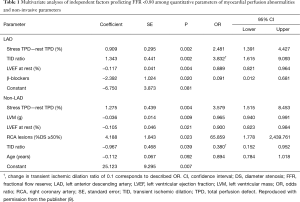
Full table
Other approaches to assess parameters that might be more appropriate to the physiological characterization of CAD than FFR are in progress. Absolute MBF quantitation, measurable in the order of mL/g/minute, which allows the non-invasive calculation of myocardial flow reserve (MFR) or CFR using stress cardiac positron emission tomography (PET) demonstrated superior risk stratification and incremental prognostic value (10,11). However, a benefit of CFR quantitation in terms of selecting patients for REV has not yet been demonstrated in randomized clinical trials. Absolute MBF quantitation using stress cardiac magnetic resonance (CMR) (12) or dynamic SPECT imaging (13) is based on a theory similar to that of cardiac PET. However, more evidence is required before this concept could be applied to routine clinical practice.
The issue then, is whether or not all patients with stable CAD should be assessed by invasive FFR. We believe that a non-invasive diagnostic modality or a combination of such approaches with highly accurate prognostic value for stable CAD that can precisely clarify the contribution of epicardial coronary stenosis to abnormal findings, might eventually resolve this issue.
Acknowledgements
The authors would thank the publisher Am J Cardiol provide the permission to use the table and figure.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Yue Liu (Associate professor, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pijls NH, van Son JA, Kirkeeide RL, et al. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993;87:1354-67. [Crossref] [PubMed]
- Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703-8. [Crossref] [PubMed]
- Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007;49:2105-11. [Crossref] [PubMed]
- De Bruyne B, Pijls NH, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001. [Crossref] [PubMed]
- Min JK, Taylor CA, Achenbach S, et al. Noninvasive Fractional Flow Reserve Derived From Coronary CT Angiography: Clinical Data and Scientific Principles. JACC Cardiovasc Imaging 2015;8:1209-22. [Crossref] [PubMed]
- Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283-91. [Crossref] [PubMed]
- Motwani M, Motlagh M, Gupta A, et al. Reasons and implications of agreements and disagreements between coronary flow reserve, fractional flow reserve, and myocardial perfusion imaging. J Nucl Cardiol 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Echavarria-Pinto M, Escaned J, Macías E, et al. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation 2013;128:2557-66. [Crossref] [PubMed]
- Tanaka H, Takahashi T, Kozono N, et al. Prediction of Flow-Limiting Fractional Flow Reserve in Patients With Stable Coronary Artery Disease Based on Quantitative Myocardial Perfusion Imaging. Am J Cardiol 2016;117:1417-26. [Crossref] [PubMed]
- Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740-8. [Crossref] [PubMed]
- Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215-24. [Crossref] [PubMed]
- Costa MA, Shoemaker S, Futamatsu H, et al. Quantitative magnetic resonance perfusion imaging detects anatomic and physiologic coronary artery disease as measured by coronary angiography and fractional flow reserve. J Am Coll Cardiol 2007;50:514-22. [Crossref] [PubMed]
- Petretta M, Storto G, Pellegrino T, et al. Quantitative Assessment of Myocardial Blood Flow with SPECT. Prog Cardiovasc Dis 2015;57:607-14. [Crossref] [PubMed]


