In March to April 2009, initial reports of infection with a novel swine-origin influenza A (H1N1)
virus emerged in Mexico and the United States. This soon manifested as a pandemic, worsened
by an eruption of cases in Australia and New Zealand by May, placing a substantial strain on intensive
care units worldwide. A recent inception-cohort study conducted in Australia and New Zealand
was published in the New England Journal of Medicine, reporting that between June and August,
2009, a total of 722 patients with confirmed 2009 H1N1 virus infection (28.7 cases per million inhabitants;
95% confidence interval [CI], 26.5 to 30.8) were admitted to an ICU in Australia or New
Zealand. Of the 722 patients, 669 (92.7%) were younger than 65 years of age and 66 (9.1%) were
pregnant. Of the 601 adults for whom data were available, 172 (28.6%) had a body-mass index > 35.
Patients infected with the 2009 H1N1 virus were in the ICU for a total of 8815 bed-days (350 per
million inhabitants). The median duration of treatment in the ICU was 7.0 days (interquartile range,
2.7 to 13.4). As of September 7, 2009, a total of 103 of the 722 ICU patients (14.3%; 95% CI, 11.7 to
16.9) had died, and 114 (15.8%) remain hospitalized (
1).
While the profile of the infection generally describes one of mild nature, with most patients being
minimally symptomatic, a significant proportion of the cohort proceeded on to develop severe respiratory
failure. These patients commonly belong to a vulnerable population with predisposing risk factors
including existing chronic respiratory disease, immune-suppression, pregnancy, obesity, chronic
cardiovascular disease and diabetes mellitus. A review of H1N1 cases in Victoria found that 20% of
all hospital admissions required intensive care management. Of this group, more than 90% had one
or more of the above risk factors (
2). Causes of the rapidly progressive respiratory failure include
pneumonitis, pneumonia and acute respiratory distress syndrome. Patients commonly present with
hypoxemia, hypercapnia or both, often refractory with conventional mechanical ventilation. Mechanical
ventilation, which is usually characterised by high airway pressures and oxygen concentrations,
often induces additional trauma exacerbating the existing lung injury. Hence in the management
of patients with H1N1-associated respiratory failure unresponsive to conventional ventilation,
extracorporeal membrane oxygenation (ECMO) ought to be considered.
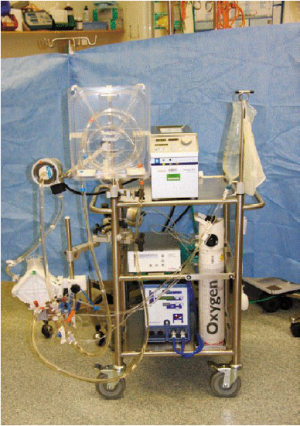
ECMO is a modified version of the cardiopulmonary bypass system (
Fig 1). It consists of the removal
of venous blood via cannulation of the femoral vein or right atrium, which is subsequently
oxygenated and returned to the circulation via the femoral artery or ascending aorta. It provides efficient
gaseous exchange at low pressure and FiO
2 ventilation, thereby minimising iatrogenic lung injury
whilst providing time for diagnosis, treatment and recovery to occur. Although ECMO was previously
associated with a high mortality risk from spontaneous haemorrhage due to the extensive anti-coagulation required, complications of ECMO have since significantly
decreased with the development of newer centrifugal
pumps, polymethyl pentene oxygenators and heparin-bonded circuits
(
3).
Of the total cohort reviewed in the Australia and New Zealand
inception-cohort study, 456 of the 722 patients (64.6%) required
mechanical ventilation, for a median of 8 days (interquartile range,
4 to 16). Of which, 53 (11.6%) were subsequently treated with ECMO,
representing 2.1 patients (95% CI, 1.5 to 2.7) per million inhabitants
(
1).
The Australia and New Zealand Extracorporeal Membrane
Oxygenation (ANZ ECMO) Influenza Investigators recently published
an exhaustive review of all Australia or New Zealand patients
who were treated with ECMO for severe H1N1-associated
ARDS in multiple centres (
4). Of the 194 patients with suspected
or confirmed influenza A (H1N1) infection, only 61 were treated
with ECMO. The median age of this cohort was 34.4 (range:
26.6-43.1) years-old, comparably older than the age group of twenties
found to be of increased susceptibility to infection (
5). Comorbidities
associated with the need for ECMO support included a
body mass index greater than 30kg/m2, asthma and diabetes mellitus.
The median interval between the onset of flu-like symptoms
and ECMO support was 9 days, while median duration of ECMO
support required was 10 (range: 7-15) days. In consideration of
their initial > 50% mortality risk (for ECMO to be indicated), the
drastically lowered mortality rate of 21% should definitely be interpreted
as a positive outcome.
The efficacy of ECMO therapy has been further reinforced by the recently published findings of the CESAR multicentre randomised
controlled trial (
6). Patients with severe but potentially reversible
respiratory failure were randomised to receive either conventional
or ECMO ventilation. ECMO patients were found with a
lower mortality rate and significantly increased survival in absence
of severe disability at 6 months. The recently published H1N1 specific
supplements in the Extracorporeal Life Support Organization
guidelines expressed the need for a lower threshold for conventional
optimal treatment to be considered inadequate, and for ECMO to
be utilised (
7). This is in contrast to the conservative approach
most clinicians currently possess, as reflected by the mere 31% utilization
in the observational study. A more liberal attitude towards
ECMO use is especially important in light of the significantly lowered
30% survival when ECMO is commenced with 7 or more
days of intubation, in comparison to the 72% survival when initiated
at 6 days of intubation (
7).
Another deterrent to ECMO support is the heavy burden it
places on intensive care services. The provision of an ECMO service
requires high levels of expertise and extensive staffing resources.
In addition, the need to transport patients to tertiary centres
equipped to provide ECMO will place great demand on retrieval
services. However, despite general perceptions of it being
unaffordable, actual cost of treatment has been found to be a mere
10% more than that for mechanical ventilation (
8). The expense of
ECMO treatment may be further minimised with strategies such as
setting up large-scale specialised treatment centres in strategic locations.
This would ensure intensive accumulation of management
experience, as well as facilitate communication and organisation
within centres, accelerating the advancement of management techniques.
As such, ECMO treatment would then be optimised, eventually
reducing the duration of ventilatory support required for recovery,
making it more cost effective.
The integration of ECMO into current treatment strategies for
H1N1-induced respiratory failure, such as antivirals, antibiotics,
mechanical ventilation and recruitment maneuvers, will significantly
improve patient outcomes. Promptness in commencement of
ECMO support is of utmost importance. Ventilatory experiences
should be widely disseminated so as to derive the most optimal
treatment logarithm for Influenza A (H1N1) respiratory failure,
minimising further damage from this unheralded attack.