Surgery of colorectal cancer lung metastases: analysis of survival, recurrence and re-surgery
Introduction
In 2012, there were 446,800 new cases of colorectal carcinoma (CRC) in Europe (1). Metastatic disease developed mostly within 3 years (2) in approximately 50% of cases: the first site of distant relapse was the liver, followed by the lung. Patients affected by metastatic CRC experienced a poor prognosis. Whenever feasible, surgery was considered an effective therapeutic option, with reported 5-year overall survival (OS) rates after radical resection ranging from 41% to 68% (3,4).
The unconfirmed predictors of survival, the lack of large randomized trial and the undefined role of metastasectomy remained the unsolved problem in the management of patients with pulmonary metastasis (MTS) of CRC (5).
The purpose of the study was to evaluate the efficacy and the feasibility of lung metastasectomy in colorectal cancer (CRC) in our institution. Moreover, we aimed at exploring prognostic factors for survival and evaluating survival pattern in patients submitted to re-resections.
Methods
We included all consecutive patients who underwent lung metastectomy for pulmonary metastases from CRC at Department of Thoracic Surgery, University of Torino, Italy—from 2004 to 2012. Data were retrospective collected from the surgical registry of our Department. Follow-up (FU) data were acquired by routine visits, telephone contacts or from administrative data (outpatient regional registry). FU was completed over July 2014. Our Institutional Review Board approved the study. Inclusion criteria for lung metastasectomy comprised locally controlled primary CRC, no evidence of extra-thoracic lesions excluding hepatic metastases, single or multiple pulmonary nodules suitable for a radical resection. Stage of primary CRC was assigned according to the Union Internationale Contre le Cancer (UICC) staging system. Pre-operative patients assessment included clinical examination, blood tests, electrocardiogram, standard chest radiograph, contrast enhanced computed tomography (CT) scan of the chest and abdomen and positron emission tomography (PET) whole-body scan. Pulmonary function tests with diffusion capacity and arterial blood gas analysis were also performed.
Type of surgical approach was selected according to the number, the location and the la laterality of the lesions; usually muscle-sparring axillary thoracotomy was the access of choice. In case of synchronous bilateral lesions, the surgical timing was tailored on each patient’s characteristic: patient with high number of metastases, larger lesions, old age or with poor pulmonary capacity was operated sequentially, at distance of one month. Complete palpation of the lung was performed in all the cases, except in case of thoracoscopic procedure. Wedge resection was accomplished to treat peripherally located pulmonary nodules; anatomical resections (segmentectomy or lobectomy) were achieved in case of multiple nodules in the same pulmonary segment or lobe, large size of the tumor or in case of a lesion located deeply in the parenchyma. Surgery was defined as radical (R0) when a complete tumor resection was accomplished, incomplete in case of microscopically (R1) or macroscopically (R2) residuals. Lymph node assessment included multiple biopsies of suspicious pulmonary and mediastinal lymph node.
Data variables and outcomes
Primary outcome was OS, calculated from the date of intervention to the date of death from any cause. Surviving patients were censored on the date of last FU. If bilateral resections were accomplished, outcomes were calculated from the date of the first surgery and resections were calculated as a single metastasectomy. Perioperative mortality was defined as any death occurred within 30 days from surgery: these events were included in the survival analysis.
According to literature, the following prognostic factors were collected: sex, gender, Eastern Cooperative Oncology Group (ECOG) performance status, Charlson Comorbidity Index (CCI) (age-adjusted), disease-free interval (DFI), primary tumor TNM stage, pre-thoracic surgery carcinoembryonic antigen (CEA) level, previous MTS in other sites, MTS number, metastases distribution, type of surgical resection, maximum lung MTS size, lymph-nodal involvement, resection status, adjuvant therapy after lung intervention.
DFI was defined as the time period between curative primary CRC surgery and metastasectomy.
Secondary analysis was performed to define the progression free survival (PFS), calculated from the date of intervention to the date of local or distant recurrence or death from any cause.
Survival in case of re-intervention and correlation between pre-operative PET scan lymph-nodal status and pathological lymph-nodal involvement were also evaluated.
Statistical analysis
Time-to-event variables were estimated using the Kaplan-Meier method and compared using log-rank test. Possible predictors of survival were investigated using the Cox proportional hazards regression model. Hazard ratios (HR) and the corresponding 95% confidence intervals (95% CI) were provided. The results of the univariate analyses were not reported in the text, in order not to lengthen the paper. The following predictors were included in the final multivariate model: advanced primary CRC pTNM stage (III–IV), age-adjusted CCI (as continuous variable), number of MTS (2–4 vs. 1 and ≥5 vs. 1), lung MTS size (≥20 mm), lymph-nodal involvement, incomplete surgery (R1-2), DFI (≥30 months) and adjuvant therapy after lung resection.
All statistical analyses were performed using SAS (version 8.2) and STATA (version 12.1).
Results
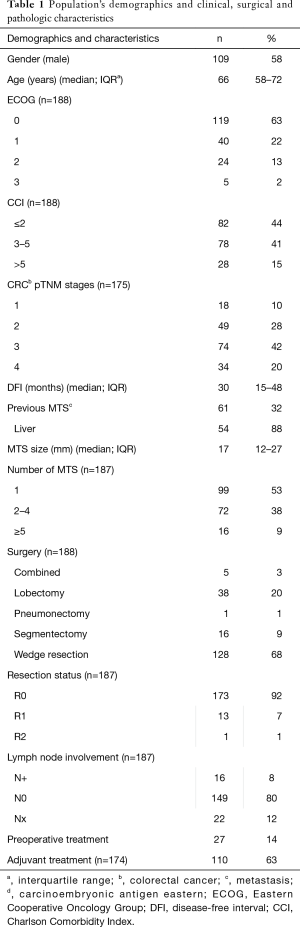
One-hundred eighty-eight consecutive cases were included in the final analysis. Population’s demographics and clinical, surgical and pathologic characteristics are showed in Table 1.
Full table
Most of patients were male (109, 58%), median age at surgery was 66 years [interquartile range (IQR), 58–72 years]. Most patients had an ECOG performance status at surgery of 0–1 (159, 85%) and an age-adjusted CCI value less than 2 (82, 44%). Thirty-three (18%) patients had a previous malignant solid tumor, other than primary CRC.
Concerning primary CRC, all patients underwent a radical resection of the primary tumor and most of them had an advanced stage cancer (III–IV; 108, 62%). After colon resection, 110 patients (63%) received adjuvant chemotherapy, with varied drugs regimens according to the different oncological team, which followed the patients. In sixty-one patients (32%) a previous MTS was observed before the lung lesion: 54/61 were liver metastases (88%). All metastases were radically treated before thoracic surgery. Median disease-free interval (DFI) was 30 months (IQR, 15–48 months).
One-hundred fifty-two patients (81%) was treated for unilateral lung metastases and 53/152 patients (35%) had multiple pulmonary nodules (range, 2–11). Thirty-six patients had multiple bilateral lesions: two patients were operated through a complete sternotomy, 34 (94%) through a two open axillary thoracotomy. Five patients were submitted to thoracoscopic resections (3%). In most of the cases wedge resections were achieved (128, 68%), in the remaining patients anatomical resection (29%; 38 lobectomy, 16 segmentectomy and 1 pneumonectomy) or combined resection (5, 3%—e.g., anatomical resection + wedge resection) were performed. Median metastases size was 17 mm (IQR, 12–27 mm). A radical resection was accomplished in 173 cases (92%) and lymphadenectomy was performed in 165 cases (88%): 16 patients (8%) had a histologically proved lymph-nodal involvement.
Five (2%) patients experienced a major complication: 2 contralateral pneumothorax, 1 pneumonia, 1 haemothorax and 1 heart failure. One perioperative death was observed.
One-hundred twenty-two patients (65%) received a complementary treatment: 110 (63%) adjuvant chemotherapy and 27 (14%) preoperative chemotherapy (14 patients both regimens).
In 179 patients (95%) PET whole-body scan was assessed: lymph node resulted positive in 10 patients. One-hundred thirty-seven node metastases were correct evaluated by PET (accuracy 86%): positive predictive value and negative predictive value were 20% and 91%, respectively.
Predictors and outcome
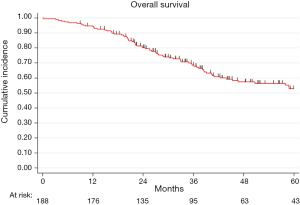
The median FU was 45 months (IQR, 30–69 months) and at the end of the study period, 77 patients died (41%). In the analyzed population, overall 2- and 5-year survival were 80% (95% CI: 73–85%) and 53% (95% CI: 44–60%), respectively (Figure S1).
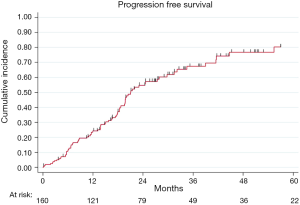
At the end of the study period, 175 patients were evaluable for relapse and 90 (51%) relapses were observed. After a R0 resection, PFS rates at 2- and 5-year were 54% (95% CI: 46–62%) and 33% (95% CI: 25–42%), respectively (Figure S2).
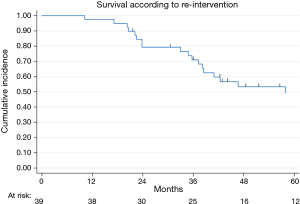
Thirty-nine relapsed patients (41%) were re-submitted to lung metastasectomy: 35 (90%) were re-operated once and 4 twice. Figure 1 showed survival in the cohort of patients submitted to re-intervention: 2- and 5-year survival after re-resection were 79% (95% CI: 63–89%) and 49% (95% CI: 31–65%), respectively.
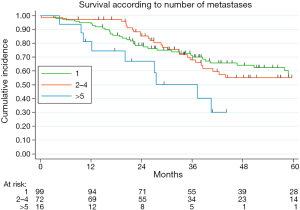
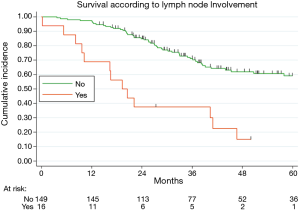
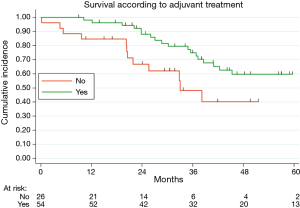
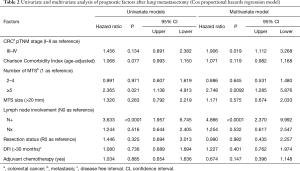
Table 2 illustrated the results of univariate and multivariate models. At univariate analysis number of resected MTS ≥5 (Figure S3, P=0.021), high CCI (P=0.077) and lymph nodal involvement (Figure 2, P<0.0001) negatively influence survival. Moreover, patients with multiple metastases submitted to adjuvant treatment showed a slight better survival (P=0.03, Figure 3).
Full table
Multivariable adjusted analysis indicated that primary CRC pathological TNM stages (P=0.019), number of resected MTS ≥5 (P=0.009) and lymph nodal involvement (P<0.0001) are independent predictors of poor prognosis.
Discussion
The first objective of this study was to evaluate the efficacy and feasibility of lung metastasectomy in CRC and factors of prognostic relevance for survival in these patients. Moreover, we aimed to define survival pattern in patients submitted to re-resection after lung recurrence.
The results of our study suggest that (I) primary CRC TNM stage, more than five resected MTS and lymph nodal involvement demonstrated to be independent predictors of survival after surgical resection; (II) survival after surgery remains comforting up to four resected MTS; (III) patients submitted to re-intervention for lung relapses showed a satisfactory prognosis; (IV) absence of pathological uptake at a preoperative PET scan was not significantly associated with absence of lymph node involvement; (V) adjuvant chemotherapy seems to have a positive effect on survival in patients affected by multiple metastases.
Prerequisites for potentially curative lung metastasectomy, first described by Thomford in 1965 (6) are widely applied today, with minor modification: (I) metastases resection deemed to be technically feasible; (II) acceptable surgical risk; (III) control of the primary tumor; and (IV) no evidence of extra-thoracic MTS. However, many issues on timing and proper indication to surgery remained opened and discussed worldwide. Actually, it is undeniable that patients undergoing surgery and redo surgery are exposed to post-operative complications. Nevertheless, in our experience morbidity is low (2%) and related with more extended resection (e.g., pneumonectomy, combined resection), in line with the data reported in other lung metastasectomy series (0–2.5%) (4,7).
In the subset of patients eligible for resection, 5-year OSs range from 25% to 68% (3). The identification of predictive factors in this sub-group is essential to select those patients who really benefit from surgery and to tailor pre- and/or post-operative strategy. To date, available evidence is still based on single center experience, often reported series over a long period of time, and few systematic reviews. Consequently, predictive factors remained controversial. Hopefully, some clarification is expected from a prospective randomized trial (8), set up to investigate the efficacy of “active monitoring with pulmonary metastasectomy” in patients with lung metastases from CRC.
Previous studies including our series show the prognostic significance of hilar and/or mediastinal lymph node involvement (4,7,9-11). Even if the role of mediastinal systematic nodal dissection remains unclear (12), our results and the bulk of previous literature supported the lymph node sampling practice.
Fluorodeoxyglucose-labeled positron emission tomography (FDG-PET) allows the detection of intra- and extrathoracic tumor deposits. Pastorino at al. illustrated the importance of PET in the preoperative mediastinal and hilar staging of pulmonary metastases, reporting a high sensitivity and accuracy (100% and 96%) (13). Contrariwise, a recent study (14) reported a high rate of unexpected lymph node disease in resected lung MTS and emphasized the value of lymph node sampling. In our series the positive predictive value of PET on detecting lymph nodal involvement results remarkably low (20%), even if negative predictive value remains high as expected (91%). In our opinion, these findings do not undervalue the value of the PET as pre-operative staging exam, but they underline the need to perform at least a selected lymph node sampling in order to eradicate a possible unidentified source of metastatic spreads and to allow a more personalized post-operative treatments and FU.
In the present study as well as previously reported in literature (15,16), pathologic stage of primary CRC had a significant impact on survival. Nonetheless, there is no definite agreement on the value of its prognostic role: the majority of authors failed to confirm a correlation between primary tumor stage and survival (4,17).
Poor survival for patient with multiple MTS has been frequently reported in literature (18,19). In our institution, multiple resection of colorectal MTS is usually achieved when feasible: the results presented in this study indicated a similar survival from 1 to 4 resected metastases. In the recent years, some studies have documented a comparable comforting survival in oligometastatic patients (9-11,20,21), probably resulting from improvements in pre-operative selection, surgical techniques and perioperative management. Although this finding deserves future validation, the significant prognostic value of the presence of more than five metastases corroborates the importance of surgery in oligometastatic disease.
Rate of relapses after resection of colorectal lung metastases is uncommonly reported in literature and remains discouraging high, ranging from 20% to 68% (4,7), with the lung as the commonest site of recurrence. In case of local relapses after lung metastasectomy, redo-surgery could be a reasonable therapeutic choice: in the last years, a number of reports in the literature documented a survival benefit after re-resection (7,10). In our experience, 5-year survival of patient submitted to repeat lung metastasectomy is encouraging (49%). Nevertheless, if only a highly selected subset of patients is eligible for pulmonary resection, re-intervention results feasible exclusively in an even more restricted patient subset. For these reasons new randomized control trials are needed to determine the true benefits of re-resection, especially because, in this setting, surgery should be compared with alternative treatment regimens [i.e., radiofrequency ablation (RFA), stereotactic ablative radiation therapy (SABR)] (22).
Finally, in our analysis, we observed an improved survival in patients with multiple metastases submitted to post-operative chemotherapy vs. surgery alone. The impact of adjuvant chemotherapy after lung metastasectomy was investigated in few studies, demonstrating a protective effect of chemotherapy on survival (23). Recently, a pooled analysis of two randomized clinical trial evaluating adjuvant chemotherapy after resection of colorectal liver or lung metastases indicated a potential positive effect of post-operative chemotherapy, but both studies were closed due to difficulties in patients’ enrolment and the benefit on OS remains undetermined (24). Future controlled clinical trials on adjuvant chemotherapy after lung metastasectomy are advisable, maybe focused on patients affected by multiple metastases.
Conclusions
Patients operated and re-operated for lung metastases from CRCs show an encouraging survival. Our results indicate that primary CRC stage; number of MTS and lymph nodal involvement are strong prognostic factors. Prognosis after surgery remained comforting up to 4 resected MTS. Furthermore, adjuvant chemotherapy seems to have a benefit on survival in patients affected by multiple metastases. Finally, precise pre-operative selection remains mandatory and according to the high rate of unidentified lymph node involvement in pre-operative setting, lymph node sampling should be advisable for a correct staging.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Institutional Review Board.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Sargent DJ, Patiyil S, Yothers G, et al. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the Accent Group. J Clin Oncol 2007;25:4569-74. [Crossref] [PubMed]
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010;375:1030-47. [Crossref] [PubMed]
- Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J Thorac Oncol 2010;5:S172-8. [Crossref] [PubMed]
- Treasure T. Pulmonary metastasectomy for colorectal cancer: weak evidence and no randomised trials. Eur J Cardiothorac Surg 2008;33:300-2. [Crossref] [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed]
- Welter S, Jacobs J, Krbek T, et al. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2007;84:203-10. [Crossref] [PubMed]
- Treasure T, Fallowfield L, Lees B, et al. Pulmonary metastasectomy in colorectal cancer: the PulMicc trial. Thorax. 2012;67:185-7. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Kim HK, Cho JH, Lee HY, et al. Pulmonary metastasectomy for colorectal cancer: how many nodules, how many times? World J Gastroenterol 2014;20:6133-45. [Crossref] [PubMed]
- Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg 2002;124:1007-13. [Crossref] [PubMed]
- Hamaji M, Cassivi SD, Shen KR, et al. Is lymph node dissection required in pulmonary metastasectomy for colorectal adenocarcinoma? Ann Thorac Surg 2012;94:1796-800. [Crossref] [PubMed]
- Pastorino U, Veronesi G, Landoni C, et al. Fluorodeoxyglucose positron emission tomography improves preoperative staging of resectable lung metastasis. J Thorac Cardiovasc Surg 2003;126:1906-10. [Crossref] [PubMed]
- Seebacher G, Decker S, Fischer JR, et al. Unexpected lymph node disease in resections for pulmonary metastases. Ann Thorac Surg 2015;99:231-6. [Crossref] [PubMed]
- Melloni G, Doglioni C, Bandiera A, et al. Prognostic factors and analysis of microsatellite instability in resected pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2006;81:2008-13. [Crossref] [PubMed]
- Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238-44. [Crossref] [PubMed]
- Blackmon SH, Stephens EH, Correa AM, et al. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg 2012;94:1802-9. [Crossref] [PubMed]
- Salah S, Watanabe K, Welter S, et al. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol 2012;23:2649-55. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Haney JC, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg 2009;87:1684-8. [Crossref] [PubMed]
- Lo SS, Moffatt-Bruce SD, Dawson LA, et al. The role of local therapy in the management of lung and liver oligometastases. Nat Rev Clin Oncol 2011;8:405-16. [Crossref] [PubMed]
- Tampellini M, Ottone A, Bellini E, et al. The role of lung metastasis resection in improving outcome of colorectal cancer patients: results from a large retrospective study. Oncologist 2012;17:1430-8. [Crossref] [PubMed]
- Filippi AR, Badellino S, Ceccarelli M, et al. Stereotactic ablative radiation therapy as first local therapy for lung oligometastases from colorectal cancer: a single-institution cohort study. Int J Radiat Oncol Biol Phys 2015;91:524-9. [Crossref] [PubMed]
- Brandi G, Derenzini E, Falcone A, et al. Adjuvant systemic chemotherapy after putative curative resection of colorectal liver and lung metastases. Clin Colorectal Cancer 2013;12:188-94. [Crossref] [PubMed]
- Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008;26:4906-11. [Crossref] [PubMed]