Overlapping meta-analyses of bioresorbable vascular scaffolds versus everolimus-eluting stents: bringing clarity or confusion?
The ABSORB (Abbot Vascular, Santa Clara, CA, USA) bioresorbable vascular scaffold (BVS) has been conceived to address some residual shortcomings of metallic drug-eluting stents (DES), including very late thrombosis and loss of vasomotion due to permanent caging of the coronary vessel. In Europe, BVSs were approved in 2011 mainly based on data from the ABSORB study, a two-stage single-arm investigation with multimodal imaging assessment including a total of 131 patients (1,2). To further investigate the device in a broader population and support European commercialization and reimbursement activities, the manufacturer initiated ABSORB II, a randomized controlled trial of 501 patients, where BVSs were tested on two surrogate co-primary endpoints (i.e., vasomotion and late lumen loss) against the cobalt-chromium XIENCE (Abbott Vascular, Santa Clara, CA, USA) everolimus-eluting stent (EES) (3). Interim 1-year results of ABSORB II have been published in 2015, showing no significant differences between BVSs and EESs, but these findings are at best hypothesis generating, due to the low statistical power of the study for clinical endpoints (4).
In the United States, China and Japan, other randomized comparisons versus EESs have been conducted to support approval by local regulatory authorities. The ABSORB III trial (N=2,008) was designed as a non-inferiority study, with a margin of 4.5% for the putative risk difference between BVSs and EESs in 1-year target lesion failure (TLF, a composite of cardiac death, target vessel myocardial infarction and ischemia-driven target-lesion revascularization) (5). This margin of non-inferiority was selected based on Food and Drug Administration (FDA) recommendations, in that it represents the 50% of the lower boundary of the 90% confidence interval of the treatment effect for EESs as compared with bare metal stents. ABSORB 3 showed a risk difference in 1-year TLF of 1.7% (7.8% in the BVS group and 6.1% in the EES group), with the 95% upper bound of the confidence interval corresponding to 3.9%, a figure below the pre-specified non-inferiority margin (6). ABSORB China was also designed under a non-inferiority assumption, but the trial was powered only for a 0.15 mm margin in the difference of 1-year in-segment late lumen loss. This resulted into a smaller sample size than ABSORB 3 (N=480), but non-usable conclusions at the clinical level. The difference in 1-year in-segment late lumen loss was 0.06 mm (0.19±0.38 mm in the BVS group and 0.13±0.38 mm in the EES group), and the upper bound of the confidence interval was just 1 mm below the non-inferiority threshold (7). ABSORB Japan used a wide non-inferiority margin for the difference in 1-year TLF (8.6%), based on an agreement with the Japanese Pharmaceutical and Medical Device Agency, which resulted in a small sample size (N=400). The trial showed a 0.4% risk difference in 1-year TLF between BVSs and EESs (4.2% in the BVS group and 3.8% in the EES group), and the upper bound of the confidence interval was 4.0% (8). The sample size of Absorb Japan was sufficient to power a test of non-inferiority for late lumen loss at 13 months, using a 0.20 mm non-inferiority margin, which ultimately showed a difference of 0.01 mm (0.13±0.30 mm in the BVS group and 0.12±0.32 mm in the EES group), with a 0.06 mm upper bound of the 95% confidence interval.
The 1-year results of the 4 ABSORB randomized trials invoke the idea of BVSs being non-inferior to EESs. Non-inferiority designs are used and perhaps abused in contemporary trials of new coronary devices, which unfortunately does not contribute to progress significantly the field of interventional cardiology (9). In the case of BVSs, one may advocate that establishing non-inferiority at 1 year is enough for a device whose benefits over metallic DESs are expected to accrue after bioresorption. The ABSORB IV trial (NCT02173379), which is currently testing the hypothesis that BVSs are noninferior (with reflex to superiority) to EESs in the landmark analysis of TLF between 1 and 5 years, will contribute to define the role of BVSs in modern practice. In the meantime, taken separately, all the ABSORB trials have limitations in the strength of their clinical conclusions. Indeed, ABSORB II and ABSORB China were not statistically powered for clinical outcomes, ABSORB Japan used a wide non-inferiority margin and had a lower than anticipated event rate, and ABSORB III was not designed to address individual endpoints or to exclude small differences in TLF.
When independent trials are not sufficient to address the effect of an intervention, meta-analyses increase the statistical power of treatment comparisons beyond that of individual studies, with the ultimate goal of informing clinical practice and guiding healthcare decisions. But what happens if a plethora of meta-analyses of BVSs vs. EESs become simultaneously available on the same topic and display mixed results? Table 1 summarizes the characteristics and results of 5 meta-analyses of BVSs versus EESs published in 2016. Stone et al. pooled 3,389 patients from the 4 ABSORB trials on a patient-level basis (10). Cassese et al. (12) and Bangalore et al. (13). combined study-level data of 3,738 patients from the ABSORB trials and two additional small investigator-driven randomized studies of BVSs versus EESs named EVERBIO 2 and TROFI 2 (15,16). Lipinski et al. also combined study-level data but included only two randomized studies (ABSORB II and TROFI 2) and a number of non-randomized comparisons (11). Finally, Kang et al. performed a network meta-analysis of 147 stent and scaffold trials, where the comparison of BVSs and EESs represents just one node of the framework, and the results reflect the combination of direct and indirect evidence estimates (14). When appraising if consistency exists in the results of overlapping meta-analyses of BVSs, a first major conundrum is that these results have not been uniformly reported for all the potential endpoints of interest. Also, the available follow up was shorter in the meta-analysis of Lipinski et al. (11), and in some cases there was a variation in endpoint definitions (i.e., myocardial infarction as opposed to target-vessel myocardial infarction; target lesion revascularization as opposed to ischemia-driven target lesion revascularization). The device-oriented clinical endpoint of TLF was appraised by only two meta-analyses (10,12) and shown to be similar between BVSs and EESs. Similarly, none of the meta-analyses displayed a difference in all-cause and cardiac death. Myocardial infarction was significantly increased only in the meta-analysis from Lipinski et al. (11), but trended towards statistical significance in the other four studies. Target-lesion and target-vessel revascularization did not differ between BVSs and EESs. Finally, a consistent finding across all meta-analyses was the approximately 2-fold increase in definite or probable device thrombosis with BVSs, which was significant in three out of five studies (11,12,14). Overlapping meta-analyses can result in a certain degree of ambiguity when they come to discordant conclusions (17). Indeed, the conclusions of the abstract of these meta-analyses also sound different, ranging between the positive outlook of Stone et al. (“BVS did not lead to different rates of composite patient-oriented and device-oriented adverse events at 1-year follow-up compared with cobalt-chromium EESs”) (10), and the negative viewpoint of Lipinski et al. (“BVS had increased definite/probable device thrombosis and myocardial infarction during follow-up compared with DES”) (11).
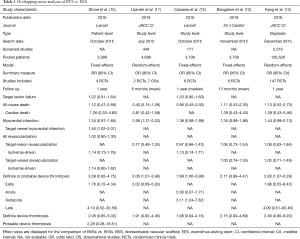
Full table
How can we reconcile all the disparate results and conclusions of the five meta-analyses of BVSs vs. EESs in view of their non-uniform eligibility criteria, and overall differences in target population analyzed, follow up and endpoint definitions? One way is to realize individual strengths and weaknesses of these studies. Patient-level meta-analyses allow better alignment of definitions and follow-up, and enable ancillary tests that would be unfeasible at the study-level. These latter include generating time-to-event curves, identifying independent prognostic factors, and testing for interaction effects. As such, the meta-analysis from Stone et al. provides the reader with unique insights—for example, over the distribution of TLF events at follow-up (i.e., with a steep rise in the first month, followed by continuous increase up to 12 months) and the detrimental impact of baseline conditions (i.e., diabetes, small vessels, and/or complex angiographic features) (10). Study-level meta-analyses such as those by Cassese et al. and Bangalore et al. are more flexible in that they can incorporate data pertaining to trials whose full datasets have not been made available (i.e., EVERBIO 2 and TROFI 2). Notably, only Cassese et al. reported on subacute thrombosis, which was significantly increased in the BVS arm (12). Bangalore et al. used 5 different pooling models and complemented their study with a trial sequential analysis indicating the lack of a strong evidence for a hypothetical 30% increase in device thrombosis with BVSs when compared with EESs, thus concluding that the current accumulated information size is underpowered to make any firm conclusions (13). Lipinski et al. included more patients than did the other meta-analyses, extending their inclusion criteria to single-arm and case-control observational studies reflecting less selected populations than in the trial setting (11). This also allowed the authors to provide summary estimates for a wide range of clinical outcomes, and to run meta-regressions on the impact of variables such as prevalence of acute coronary syndromes in the study population and date of study initiation. Finally, the network meta-analysis approach chosen by Kang et al. permitted to incorporate the direct evidence from the available trials of BVSs vs. EES and the indirect evidence from bare metal stents and DESs trials using common comparators. This enabled a consolidated ranking of contemporary coronary devices for the outcome of 1-year definite or probable thrombosis, with BVSs positioned at the lower end of the safety spectrum, at the same level of paclitaxel-eluting stents and bare metal stents (14).
In conclusion, which of the meta-analyses published so far is the most applicable to the important clinical question of the efficacy and safety of BVSs in current practice, and which one is the most methodologically sound? At this early stage of data collection (i.e., with only ≤1-year data available in most BVSs studies), and because judgment inevitably involves assigning subjective weights to pros and cons of each meta-analytical approach, the answer may be arbitrary. The reader may personally refer to published methods and checklists to map the quality of the 5 meta-analyses described in this article, and come to a personal conclusion (17).
An FDA panel has recently reached a consensus on the fact that BVS is an effective treatment and most panelists felt that the benefits of scaffolds outweigh the risks (18). Residual skeptics and purists will contend that even meta-analyses of BVSs vs. EESs have not reached the sufficient information size to address important residual safety and efficacy questions, particularly at long-term. Indeed, these studies cannot rule out (but also cannot conclusively demonstrate) that BVSs increase thrombosis and myocardial infarction compared with best-in-class DESs at one year, but the similar risk of TLF is reassuring and supports the use of BVSs in current practice for selected patients and lesions.
To reflect the evolving knowledge in the field, BVSs meta-analyses will continue to be regularly updated as new studies become available. When preparing and submitting a new meta-analysis, the authors should take responsibility for trying to advance meaningfully the field and fairly evaluate the added value of having a new publication on the same topic. Similarly, peer reviewers and editorial boards should carefully evaluate the incremental qualities of new meta-analyses under review, to prevent the proliferation of overlapping meta-analyses bringing more confusion than clarity.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Yue Liu (Associate Professor, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: Speaker’s honoraria from Abbott Vascular, AstraZeneca, Bayer, Daiichi Sankyo, Aspen and Stentys.
References
- Onuma Y, Dudek D, Thuesen L, et al. Five-year clinical and functional multislice computed tomography angiographic results after coronary implantation of the fully resorbable polymeric everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB cohort A trial. JACC Cardiovasc Interv 2013;6:999-1009. [Crossref] [PubMed]
- Serruys PW, Onuma Y, Garcia-Garcia HM, et al. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention 2014;9:1271-84. [Crossref] [PubMed]
- Diletti R, Serruys PW, Farooq V, et al. ABSORB II randomized controlled trial: a clinical evaluation to compare the safety, efficacy, and performance of the Absorb everolimus-eluting bioresorbable vascular scaffold system against the XIENCE everolimus-eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions: rationale and study design. Am Heart J 2012;164:654-63. [Crossref] [PubMed]
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385:43-54. [Crossref] [PubMed]
- Kereiakes DJ, Ellis SG, Popma JJ, et al. Evaluation of a fully bioresorbable vascular scaffold in patients with coronary artery disease: design of and rationale for the ABSORB III randomized trial. Am Heart J 2015;170:641-651.e3. [Crossref] [PubMed]
- Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-Eluting Bioresorbable Scaffolds for Coronary Artery Disease. N Engl J Med 2015;373:1905-15. [Crossref] [PubMed]
- Gao R, Yang Y, Han Y, et al. Bioresorbable Vascular Scaffolds Versus Metallic Stents in Patients With Coronary Artery Disease: ABSORB China Trial. J Am Coll Cardiol 2015;66:2298-309. [Crossref] [PubMed]
- Kimura T, Kozuma K, Tanabe K, et al. A randomized trial evaluating everolimus-eluting Absorb bioresorbable scaffolds vs. everolimus-eluting metallic stents in patients with coronary artery disease: ABSORB Japan. Eur Heart J 2015;36:3332-42. [Crossref] [PubMed]
- Byrne RA, Kastrati A. Drug-eluting stent trials: too much non-inferiority, too little progress? Lancet 2014;383:386-8. [Crossref] [PubMed]
- Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet 2016;387:1277-89. [Crossref] [PubMed]
- Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold Thrombosis After Percutaneous Coronary Intervention With ABSORB Bioresorbable Vascular Scaffold: A Systematic Review and Meta-Analysis. JACC Cardiovasc Interv 2016;9:12-24. [Crossref] [PubMed]
- Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537-44. [Crossref] [PubMed]
- Bangalore S, Toklu B, Bhatt DL. Outcomes with bioabsorbable vascular scaffolds versus everolimus eluting stents: Insights from randomized trials. Int J Cardiol 2016;212:214-22. [Crossref] [PubMed]
- Kang SH, Chae I, Park J, et al. Stent Thrombosis with Drug-Eluting Stents and Bioresorbable Scaffolds: Evidence from a Network Meta-Analysis of 147 Trials. JACC: Cardiovasc Interv 2016;9:1203-12. [Crossref] [PubMed]
- Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol 2015;65:791-801. [Crossref] [PubMed]
- Sabaté M, Windecker S, Iñiguez A, et al. Everolimus-eluting bioresorbable stent vs. durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction: results of the randomized ABSORB ST-segment elevation myocardial infarction-TROFI II trial. Eur Heart J 2016;37:229-40. [Crossref] [PubMed]
- Osnabrugge RL, Capodanno D, Cummins P, et al. Review and recommendations on the current practice of meta-analyses: a guide to appraise the evidence. EuroIntervention 2014;9:1013-20. [Crossref] [PubMed]
- Serruys PW. Observations from the recent FDA Circulatory System Devices Panel meeting on bioresorbable vascular scaffolds. EuroIntervention 2016;11:e1569-70. [Crossref] [PubMed]


