When size matters: changing opinion in the management of pleural space—the rise of small-bore pleural catheters
Introduction: historical perspectives
Pleural or chest tubes are drains placed into the pleural space surgically or percutaneously to evacuate fluid or air.
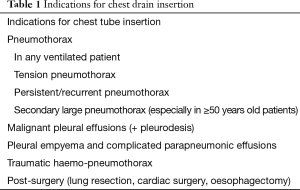
Tube thoracostomy is usually the first step to treat several thoracic/pleural conditions such as pneumothorax, pleural effusions, haemothorax, haemo-pneumothorax and empyema (Table 1). Hippocrates is known to be the first who drained the pleural space with a metal tube, treating an empyema about 2,400 years ago (1).
Full table
In the early 1990s the use of large postsurgical drains was extensively accepted, especially for the treatment of pleural empyema caused by the 1917 pandemic influenza. The “empyema commission”, created ad hoc, recommended an immediate drainage of any streptococcal pus, as the standard surgical care (2).
Lung function restoration was the primary goal of thoracic wound treatment during both the Second World War and the Korean one: emergency tube thoracostomy became extremely frequent in haemothorax and tension pneumothorax treatment. Furthermore, the drain was connected for the first time to a two-bottle water seal suction system (3). Rapidly, synthetic ones, more flexible and easy to place, replaced metal tubes and modern three chamber thoracic drain, for a more efficient suction, were employed since 1952 (4).
By the 1980s new, flexible and plastic drains were widely used: they ranged between 6 and 40 French (F) in size (5). The smaller ones (≤20 F) were commonly used in children, the bigger in adults, since it was believed that smaller drains were less effective in adult medicine, being more prone to the risk of obstruction.
In the last two decades, small-bore chest tubes (SBCT) have gained increasing popularity. Usually placed using the Seldinger technique, sometimes under radiological guidance, they were initially used to drain abdominal collections and subsequently also pleural effusions. SBCTs quickly gained popularity between surgeons, pulmonologists and oncologists, being easier to position and characterized by less pain to the patient, becoming the actual standard of care in several clinical conditions.
The aim of this paper is to review the current clinical indication for chest drain placement in pleural/thoracic diseases, comparing the effectiveness of small-bore and large-bore ones.
What size chest drain?
Chest drains are usually divided into size categories according to their size: large-bore (≥20 F) or small-bore (≤20 F). F is a standardized unit of measurement which was proposed by the French surgical instrument maker Joseph-Frederic-Benoit Charrière, in 1860. F refers to the outer diameter of a cylindrical tube and it is equivalent to 0.333 mm.
Like all cylindrical tubes, pleural drains too obey to Poiseuille’s law and the Fanning equation.
The Fanning equation (ν=π2r5P/fl, were ν is the flow, r the radius, l the length, P the pressure and f the friction factor) regulates the moist gas (such as in a pneumothorax) or liquid with turbulent flow through the tube (6). The Poiseuille’s law (ν=πr4.ΔP/8.ὴL, were ν is the flow, r the radius, ὴ the liquid’s viscosity, L the tube’s length and ΔP the difference between the tube’s ends) regulates the flow of corporeal fluids.
The internal diameters (bore), which may vary according to the manufacturer and the length of the tube, are obviously key determinants of pleural fluid flow (including blood and pus). Therefore, for an appropriate selection of the chest drain both the quality of the drained material as well as its formation rate should be taken in account. If we consider viscous fluids rapidly generated (such as blood in a traumatic patient), a large-bore chest tube (LBCT) seems to be more effective rather than a small-bore one, which is, contrariwise, adequate when a similar volume of air is produced (e.g., pneumothorax).
An experimental study compared the drainage capacity of a 19-F vs. a 28-F tube in vivo and in vitro, showing that the larger one had an in vitro capacity 9 times higher than the other, whilst, in vivo, both tubes had the same drainage capability over time (7). Similar results were also found by Park and Colleagues, who compared the catheters drainage time according to their size, taking into account the characteristics of fluid, including viscosity (8). In their study, the authors observed that the tube’s size was significantly different only for catheters smaller than 8 F, while it was not so for the larger ones. Furthermore, the size of the drain fenestrations should be considered for the tube drainage capability, but at present no studies evaluating this factor are available in literature.
Large-bore drains
Traditionally, large-bore tubes are employed whenever a high-risk of drain obstruction is expected, such as in case of empyema or active bleeding, even if this fact has never been demonstrated by randomized clinical trials. The presence of multiloculated pleural collections with high viscosity liquid, typical of stage II–III pleural empyema, make the simple tube thoracostomy often ineffective, and a surgical approach is therefore necessary.
Furthermore, the 2008 Advanced Trauma Life Support Recommendations (ATLS) report that a large tube must be considered to drain a post-traumatic haemothorax (9).
Traumatic pneumothorax, especially if the patient is mechanically ventilated, requires the placement of a chest tube, given the potential need of air and/or blood evacuation: a large-bore tube (ideally ≥28 F) was recommended by some authors (10,11).
LBCTs may be inserted using both the trocar technique (Figure 1A) or a by blunt dissection (Figure 1B). Chest tube insertion is a surgical manoeuvre, which has potential risks. As reported by Harris and Colleagues (12), during the period between 2003 and 2008, an overall of 17 fatalities caused by chest tube insertion were observed in the UK, the majority of which were due to the tube insertion into another organ. A direct injury to a surrounding structure is a potential risk intrinsic to all invasive procedures, and its occurrence might be more frequent if a large-bore drain is employed (13). Also Havelock and Colleagues (14) reported that the incidence of injuries (1.4% vs. 0.2%) and malposition (6.5% large-bore vs. 0.6% small-bore) was significantly higher for LBCTs.
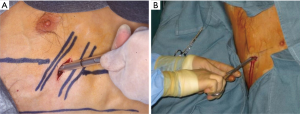
The most common drawbacks of LBCT are pain, which is directly related to the traumatic insertion of the tube through the intercostal space, and its size. In fact, several recent studies support the concept that LBCTs are directly associated with higher thoracic pain (14-17). Moreover, the risk of infection appears to be higher when a large-bore drain is used, especially for prolonged placements (18).
Small-bore drains
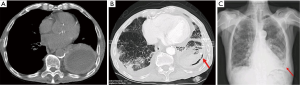
If large-bore tubes are characterized by the risk of injury at the time of insertion and thoracic pain, the intrinsic potential risk of small-bore ones are obstruction, displacement, kinking, accidental release from the insertion point and rupture (the latter with a high-risk of losing the drain into the chest cavity) (Figure 2). Historically, the reported blockage range for SBCTs was 8.1% compared to 5.2% for LBCTs (19). Sometimes, blockage requires tube removal and subsequent replacement, with pain and discomfort for the patient.
Kinking, especially in the site of chest insertion, is another possible complication of SBCTs (Figure 2A), which is obviously less frequently observed with large-bore ones.
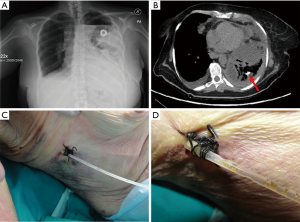
Misplacement (Figure 3A,B) is another important concern (20-22) and drain re-insertion is often required. The in situ stay of the tube for several days may be cause of its release from the chest wall insertion point (Figure 3C,D): pleural fluid leakage or pneumothorax are the most common consequences.
Usually, complications occur less frequently if the drain is placed by expert operators (senior staff Physicians) or under radiological/ultrasonographic guidance (20). There is no significant difference in complication rates when the chest tube is inserted using the Seldinger technique or through a blunt dissection, as reported by Maskell and co-authors in a large multicenter study (23).
Adherence to the existing guidelines (14,24) and a proper personal clinical experience may reduce the risk of complications during chest drain placement.
New chest drains available in the daily clinical practice
In our Department the selection policy to drain patients with acute post-traumatic haemothorax or frank/complicated empyema (stage II–III) includes the use of large-bore chest drains (24 to 32 F), evaluated on a case-by-case basis and according to the surgeon’s preference. We use small-bore tubes (8 to 20 F) to treat pneumothorax (primary or secondary), malignant or chronic pleural effusions and uncomplicated empyema (stage I).
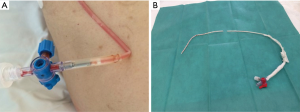
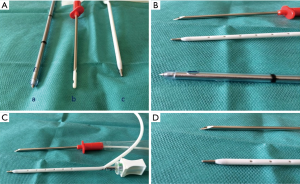
Our drain armamentarium include Argyle tubes (Covidien, Mansfied, MA, USA), Pleurocath (Prodimed, Neuilly-enThelle, France) and UNICO Forty (Redax, Poggiorusco, Italy) (Figure 4). In case of chronic malignant pleural effusions or entrapped lung, we use indwelling pleural catheters (PleurX, Carefusion San Diego, CA, USA) with excellent results in symptomatic dyspnea relief as well as its management in the outpatient clinic setting.
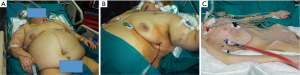
In case of localized pleural collections, we generally place 12–14 F pigtail drains (UNICO Multi, Redax, Poggiorusco, Italy) under CT-scan or ultrasonographic guidance (Figure 5). Ultrasonography was shown to be a safe technique for tube placement, with low procedure-related complication rate (25). Major advantages of the ultrasonographic guidance include the absence of radiation, low cost, the possibility to perform the procedure at the patient bedside as well as a shorter examination time, if compared to the CT. Furthermore, transthoracic ultrasonography is very useful to localize and monitor pleural collections.
SBCT is chosen according to the pathology, the type of fluid to evacuate and, particularly, patient’s habitus (Figure 6). An easy insertion technique is obviously a decisive factor in the drain’s choice: the Unico’s advantage compared to other SBCTs is intrinsic in its characteristics (Figure 4). The Verre’s needle makes the drain introduction into the chest wall safer, since the risk of iatrogenic lung injury is low. In fact, the top of the needle is covered with a blunt surface when it passes through the chest wall: the chest wall penetration is shown by a green safety indicator, which is located onto the needle knob. For such reasons, Unico’s use has increased in time along with the improvement of the surgeons’ learning curve in our Department.
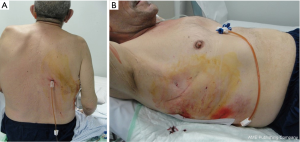
Moreover, the presence of a 20-cm external silicon extension makes possible its placement posteriorly without potential kinking or tube dislocation and allowing, at the same time, a good drainage (Figure 7). Pleural fluids are directly drained through the catheter, without the need to move the patient, hence avoiding comfortless and painful positions during the hospitalization. Moreover, this extension avoids a prolonged bed immobilization, permitting the patient to move around the bed (if electronic suction devices are unavailable) and to perform a proper respiratory physiotherapy, resulting also in an overall improvement of patient’s quality of life. This is particularly appreciated when patients with severe COPD and secondary pneumothorax are drained (Figure 8).

Moreover, Unico demonstrated to be effective also in slurry talc pleurodesis for malignant pleural effusions, in patients unfit for a traditional thoracoscopic talc insufflation because of several important comorbidities (26). Catheter’s small size and characteristics (size, resistance, anti-kinking polyurethane material, capability to conform to patient’s chest anatomy) have a strong impact on patients, in terms of tolerability and pain, especially when a long-term permanence in the chest is required. Catheter’s material reduces the risk of potential skin decubitus (more frequent when a LBCT is placed), wound infection or cutaneous bleeding.
We found that tube displacement was frequently observed when other SBCT available in our Department were used; replacement of the catheter was necessary in all cases. The rupture of the catheter was observed twice (Pleurocath) and those patients needed a thoracoscopic procedure to remove the fragments that were lost in the pleural space. The same problem was previously reported in literature (27).
Finally, radiopacity is another very important intrinsic characteristic of the chest tube since it lets a better identification of the drain into the pleural space at the plain chest X-ray. Argyle tubes have a radiopaque line along the tube side, and some holes are placed proximal to the line itself.
In conclusion, the use of SBCTs continues to increase in time; they are effective for the treatment of several pleural conditions such as pneumothoraxes, malignant/chronic effusions and simple uncomplicated empyemas. In case of active, post-traumatic haemothorax or complicated empyema, LBCT placement is still recommended. Post-insertion pain is reduced with the use of small-size drains. The risk of tube dislocation may be reduced by its placement under radiological/ultrasonographical guidance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Christopoulou-Aletra H, Papavramidou N. "Empyemas" of the thoracic cavity in the Hippocratic Corpus. Ann Thorac Surg 2008;85:1132-4. [Crossref]
- Churchill ED. Wound surgery encounters a dilemma. J Thorac Surg 1958;35:279-90.
- Monaghan SF, Swan KG. Tube thoracostomy: the struggle to the "standard of care". Ann Thorac Surg 2008;86:2019-22. [Crossref]
- Howe BE Jr. Evaluation of chest suction with an artificial thorax. Surg Forum 1951.1-7.
- Miller KS, Sahn SA. Chest tubes. Indications, technique, management and complications. Chest 1987;91:258-64. [Crossref]
- Birath G, Swenson EW. Resistance to air flow in bronchospirometric catheters. J Thorac Surg 1957;33:275-81.
- Niinami H, Tabata M, Takeuchi Y, et al. Experimental assessment of the drainage capacity of small silastic chest drains. Asian Cardiovasc Thorac Ann 2006;14:223-6. [Crossref]
- Park JK, Kraus FC, Haaga JR. Fluid flow during percutaneous drainage procedures: an in vitro study of the effects of fluid viscosity, catheter size, and adjunctive urokinase. AJR Am J Roentgenol 1993;160:165-9. [Crossref]
- American College of Surgeons. ATLS: advanced trauma life support for doctors. 8th edition. Chicago: American College of Surgeons, 2008.
- Baumann MH. Non-spontaneous pneumothorax. In: Light RW, Lee YC, eds. Pleural Disease: An International Textbook. London: Arnold Publishers, 2003;464-74.
- Despars JA, Sassoon CS, Light RW. Significance of iatrogenic pneumothoraces. Chest 1994;105:1147-50. [Crossref]
- Harris A, O'Driscoll BR, Turkington PM. Survey of major complications of intercostal chest drain insertion in the UK. Postgrad Med J 2010;86:68-72. [Crossref]
- Yamaguchi M, Yoshino I, Kameyama T, et al. Use of small-bore silastic drains in general thoracic surgery. Ann Thorac Cardiovasc Surg 2007;13:156-8.
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii61-76. [Crossref]
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010;137:536-43. [Crossref]
- Terzi A, Feil B, Bonadiman C, et al. The use of flexible spiral drains after non-cardiac thoracic surgery. A clinical study. Eur J Cardiothorac Surg 2005;27:134-7. [Crossref]
- Clementsen P, Evald T, Grode G, et al. Treatment of malignant pleural effusion: pleurodesis using a small percutaneous catheter. A prospective randomized study. Respir Med 1998;92:593-6. [Crossref]
- Parulekar W, Di Primio G, Matzinger F, et al. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest 2001;120:19-25. [Crossref]
- Collop NA, Kim S, Sahn SA. Analysis of tube thoracostomy performed by pulmonologists at a teaching hospital. Chest 1997;112:709-13. [Crossref]
- Davies HE, Merchant S, McGown A. A study of the complications of small bore 'Seldinger' intercostal chest drains. Respirology 2008;13:603-7. [Crossref]
- Cafarotti S, Dall'Armi V, Cusumano G, et al. Small-bore wire-guided chest drains: safety, tolerability, and effectiveness in pneumothorax, malignant effusions, and pleural empyema. J Thorac Cardiovasc Surg 2011;141:683-7. [Crossref]
- Fysh ET, Smith NA, Lee YC. Optimal chest drain size: the rise of the small-bore pleural catheter. Semin Respir Crit Care Med 2010;31:760-8. [Crossref]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref]
- Laws D, Neville E, Duffy J, et al. BTS guidelines for the insertion of a chest drain. Thorax 2003;58 Suppl 2:ii53-9. [Crossref]
- Liu YH, Lin YC, Liang SJ, et al. Ultrasound-guided pigtail catheters for drainage of various pleural diseases. Am J Emerg Med 2010;28:915-21. [Crossref]
- Filosso PL, Sandri A, Felletti G, et al. Preliminary results of a new small-bore percutaneous pleural catheter used for treatment of malignant pleural effusions in ECOG PS 3-4 patients. Eur J Surg Oncol 2011;37:1093-8. [Crossref]
- Paddle A, Elahi M, Newcomb A. Retained foreign body following pleural drainage with a small-bore catheter. Gen Thorac Cardiovasc Surg 2010;58:42-4. [Crossref]