Conservative management of empyema-complicated post-lobectomy bronchopleural fistulas: experience of consecutive 13 cases in 9 years
Introduction
Bronchopleural fistula (BPF) is an infrequent but potentially life-threatening complication after pulmonary surgery. The incidence of BPF is 1.3–34.3% with related mortality rate ranges from 0.7% to even 67% (1-5). Aspiration pneumonia and subsequent respiratory distress are the main causes of death. For instance, in Sirbu and colleagues’ study (4), 4 out of 22 (18.2%) patients died of acute respiratory distress syndrome (ARDS). Sonobe and associates (6) documented 2 out of 10 (20%) patients dying of BPF-related complications.
For empyema-complicated BPFs, treatment has been especially a big challenge. Previous experiences had commonly focused on fistula closure in the first step with an aim to protect the infected chest cavity from respiratory conduits and to avoid further infection dissemination and pneumonia. Chest cavity cleanup using antibiotics or disinfectants was considered in the second step to create an aseptic residual chest cavity. However, all these maneuvers were associated with certain failure rate (5,7).
In this study, we report our 9-year experiences with managing post-lobectomy BPFs by an improved method without surgical fistulas repair, investigating the efficacy and safety of this treatment.
Methods
Patients
Present study enrolled a consecutive series of patients who developed bronchopleural fistula (all bronchial stump fistulas) with concomitant empyema after lobectomy or bi-lobectomies from September 2006 to June 2015 in a single treatment group. The initiation of conservative procedure in the first case, other than conventional surgical intervention, was ascribed to the following reasons: (I) re-operation was predictably difficult due to intense adhesions; (II) sleeve bronchial resection and reconstruction was the only choice of repair due to the short bronchial stump; (III) in aim to wrap the new anastomosis, the free muscle flap resected from chest wall will cause additionally invasion; (IV) the localized empyema might be amplified to the entire thorax due to adhesion dissection if re-operated; (V) predictably prolonged air-leak after pleural adhesion dissection; (VI) possible failure rate of fistula repair and empyema control after all these challenging manipulations; and (VII) the patient had good general condition requesting conservative treatment. Later, with the favorable outcome of this first case, we realized that such a conservative treatment might be an alternative option. Subsequently, we designed the present study. All enrolled patients fitted with all of these conditions but NO. 2, which was too advanced for most cases. Actually, we believed that this treatment could be succeeded in such patient, it would also be curative in cases less advanced.
For patients met the criteria of our study, we showed them not only the options of therapy including re-operation, endoscopic closure, and conservative treatment, but also advantages and disadvantages of each therapy. In the end, all enrolled patients chose the conservative therapy. The Ethics Committee of Shanghai Pulmonary Hospital approved this study, and consents were obtained from all enrolled patients.
Diagnosis of BPF and empyema
BPF was diagnosed based on patients’ clinical presentations, computed tomography (CT) findings, and bronchoscopic manifestations. Post-operative new onset symptoms including, watery, purulent or bloody sputum, shortness of breath, and fever, were highly alerting. BPF was clinically diagnosed if subsequent CT images showed a new appearance of air-fluid level and residual cavity next to the bronchial stump. The fistula might be directly visible as a bronchial dehiscence under bronchoscopy, or an apparent continuation of a bronchus or the lung parenchyma to the pleural space on CT examination. Massive and consistent gas escape after chest tube placement provided a final confirmation of BPF. Co-existing aspiration pneumonia of the remaining lung was highly suggestive of dissemination of pleural fluid. The BPFs were then categorized as early and late types according to criteria suggested by Algar et al. (8). Early BPFs were defined as those that occurred within 30 days after lobectomies. Late BPFs refer to those beyond this time limit. Diagnosis of empyema was based on examinations of the pleural effusion. Specifically, either massive white blood cells (WBC) in the effusions (nonspecific infection) or positive culture results of the fluid contributed to the diagnosis.
Initial evaluation and general management
All patients underwent thorough evaluation of general conditions and comorbidities before fistula management. Empirical antibiotics, covering mainly Gram-negative bacteria, were administered intravenously instantly and daily. Scope and severity of co-existent pneumonia, together with possible respiratory distress, were also evaluated. Mechanical respiratory support, either by non-invasive BiPAP or ventilation with tracheal intubation, was initiated when the criteria of respiratory dysfunction were met. Co-morbidities were re-evaluated and treated as well. Residual pleural cavity and empyema were evaluated and drained as described below. Sputum and pleural effusions were repeatedly sent for cytological examination and bacterial cultures.
Tube drainage
Upon confirmation of BPF, chest tubes were placed immediately. For early BPFs, since adhesions are usually minimal, and the remnant lungs are somewhat deflated to a variable degree, 2 22# chest tubes could be easily placed in routine flavor: 1 tube was inserted to chest apex area for air exhaust while the other was placed at the lowest position of the cavity for liquid discharge. For late BPFs with localized empyema after lower lobectomies, routine intubation is usually impossible: the residual cavity commonly resides at the para-vertebral region so that no optimal intubation passage could be found from anterior or lateral body side. In this situation, pig-tail cannula or deep venous cannula provide the solution (Figure 1). Under CT guidance, the cavity could then be drained with one or multiple small tubes, which were inserted directly from patients’ back into the cavities. The softness of cannula assures patients’ comfort even when they sleep on the back. For late cases, a soft 18# or 22# tube was preferred after upper lobectomies: the cavity is usually located at the anterior-upper area of thorax, and the tube tip was better when being placed near the fistula at the bottom of the cavity. In cases of multilocular or thickened empyema, debridement, a technique of drainage through the bed of rib, was performed. After adequate debridement of empyema, chest tubes would be placed for drainage and lavage.
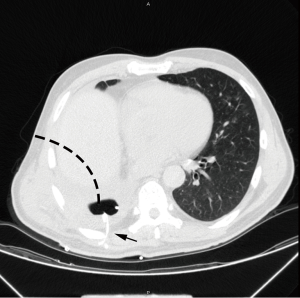
Lavage of infected cavity
The indications of empyema lavage were (I) thick inadequately drained pus and (II) uncontrolled empyema despite a well-positioned drainage tube, as verified by bacterial culture or white blood cell count of the effusions. The purpose of lavage was to decrease bacteria number to the greatest degree and to clean the cavity. Normal saline was always advocated over antibiotics and disinfectants, considering its efficacy and unexpected bronchial dissemination. The volume and frequency of irrigation depended on severity of pleural infection, and it was usually administered 2–3 times with 500 mL solution of NS and antibiotics or disinfectants a day. To ensure safety, several essential prerequisites had to be met: (I) pneumonia had to be under control; (II) the patient was able to expectorate the little amount of saline leak; (III) the patient was requested to lean on affected side and keep fistula on top of the cavity when performing irrigation; and (IV) irrigation had to be slow and cautious to prevent pulmonary aspiration. Such maneuver ended when WBC count of the pleural exudates reached normal level.
Postural drainage
Patients were educated to perform voluntary postural drainage once they had improved general condition, and able to expectorate effectively. The additional prerequisites included complete drainage of the chest cavity and daily drainage of less than 30 mL. Such small amount of chest drainage could certainly assure the safety of postural drainage without possible pus dissemination and resultant pneumonia. Different body position was adopted according to a specific fistula location and pleural cavity position. Commonly, patients were asked to lie on healthy side, just opposite to the body position when irrigation was performed (affected side). Either head-up or head-down position was advocated conditionally, based on the cavity being subsequent to prior upper or lower lobectomies. Patients were required to perform postural drainage position 3–6 times each day for at least 10 minutes each time until there were no sputum expectoration.
Removal of chest tubes
Chest tube was removed when postural drainage dominated and tube drainages revealed no effusion. In detail, the indications of tube removal were: (I) no symptom and sign of either empyema or aspiration pneumonia, as demonstrated by physical and radiological examinations; (II) the cavity could completely drained simply by postural drainage after tentatively clipping the chest tube for 24 hours and no pleural effusion retained under CT examination; and (III) the patient agreed to sustain postural drainage for additional several months. Pulmonary adhesions usually precluded remnant lung collapse, and thus tiny air-leak was not the contraindication for extubation. The patients, however, might still be mildly symptomatic, with little but limited expectorations.
Adjuvant treatment for the primary diseases
The patients were referred to the treatment of the primary diseases when aspiration pneumonia was controlled and the cavity was completely drained. Anti-fungus therapy was administered in cases of pathologically proved pulmonary aspergillosis. Even if the fistula remained open and tube drainage had purulent discharge, chemotherapies could be scheduled for cancer patients if PS score evaluation was acceptable and when there was no pneumonia.
Follow-up
Within the first 3 months after the discharge, the patient was required to have chest CT every month to ascertain complete drainage of the cavity. Body position and frequency of postural drainage were adjusted until no fluid remained in the cavity. The patients were asked to continue postural drainage for additional 3 months after symptoms were completely relieved, including expectoration. Afterwards, routine follow-up was scheduled as outpatient visit every 6 months. The fistula was considered clinically cured if one of the following criteria was met: (I) diminished pleural cavity; (II) confirmation of heal of dehiscence by bronchoscopy; and (III) patients were completely asymptomatic when resorted to normal life, despite of a small, stable, and clean cavity on CT scan. At the time of most recent follow-up, patients’ survival information was recorded as well.
Statistics
Patient demographics and outcomes were analyzed using descriptive statistics. Mean number ± standard deviation and count were used to describe continuous data and categorical data, respectively.
Results
General information
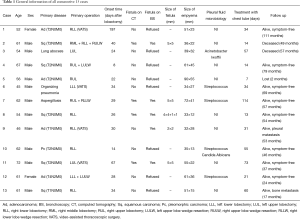
Thirteen consecutive patients, 11 males and 2 females, were included in present study (Table 1). The mean age was 57.92±7.75 years (range, 45–72 years). The primary diseases were adenocarcinoma (30.8%, 4/13), squamous carcinoma (38.5%, 5/13), pleomorphic carcinoma (7.7%, 1/13), lung abscess (7.7%, 1/13), organizing pneumonia (7.7%, 1/13), and aspergillosis (7.7%, 1/13). Two patients (15.4%, 2/13) had diabetes mellitus. Other comorbidities included chronic obstructive pulmonary diseases (2/13, 15.4%) and hypertension (3/13, 23.1%). There were 5 (38.5%) right lower lobectomies, 1 (7.7%) right middle-lower bi-lobectomy, 3 (23.1%) right upper lobectomies, 2 (15.4%) left upper lobectomies, and 2 (15.4%) left lower lobectomies. For lung cancer cases, systemic lymph node dissection harvested 139 nodes of which 5 were positive. During these lobectomies, the bronchial stumps were closed with staplers in 8 cases and 5 were sutured with 4-0 absorbable ties. None of the bronchial stumps had been enwrapped with adjacent pedicled tissues.
Full table
BPF and concomitant empyema
BPFs occurred between 8 days and 197 days (mean, 42.54±48.48 days) postoperatively, including 8 early BPFs and 5 late BPFs. Ten patients had acute presentations, including sudden watery sputum, irritable cough, shortness of breath, and hemoptysis. No one had acute respiratory distress and did not require mechanical respiratory support. On CT examinations, newly appeared air-fluid level in the residual pleural cavity and concomitant pneumonia were obviously visible in all cases, while fistula connecting bronchus or the lung parenchyma to the pleural space were found in 4 patients. Moreover, bronchial fistula manifesting as bronchial stump dehiscence due to staple split was confirmed with bronchoscopies in 5 patients with sizes ranged from 1×1 to 5×5 mm2 (Figure 2). Pneumonia was prominent in the lower lung field, with ipsilateral single lobe involvement in 6 cases and more extensive in 7 cases. Two patients had prominent lung collapse in the 8 early-fistula cases. On chest drainage, multiple types of bacteria, including nonspecific infection, Streptococcus, Candida Albicans, and Acinetobacter lwoffii were demonstrated in the pleural exudates.
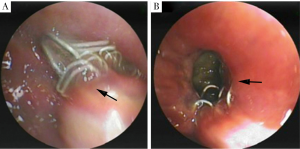
Drainage
Systemic symptoms of infection resolved quickly after chest tube drainage. In 2 patients, empyema debridement was required before tube drainage, since CT images showed suspected solidification material scattering in the cavity and there was little plural effusions on needle puncture. In 3 cases with partial lung collapse, 2 22# chest tubes were placed. In one patient with difficult chest intubation, 2 pig-tail soft tubes were utilized. The rest patients were administrated with combination of chest tube and pig-tail. In 8 patients, normal saline lavage started on mean time of 6.0±4.54 days (range, 2–15 days) after tube placement. No pneumonia execration had developed. Lavage lasted 2–45 days (mean, 19.75±15.39 days) until the pleural fluid revealed a normal WBC count. With 3 patients (cases 7, 10, 11) with multilocular or thickened empyema were discharged with chest tube inside, other patients have the tube removed before discharged. Tube drainage lasted from 7 to 114 days (mean, 40.54±30.49 days) in this cohort.
The patients were educated to practice postural drainage on mean time of 66.92±21.75 days (range, 30–90 days) after chest tube drainage. None of the patients experienced pneumonia recurrence or hemoptysis subsequent to granuloma formation in the cavity.
Diminishment of empyema cavity
In all 5 patients with bronchoscopically visible fistulas (5/13), the pleural cavity completely disappeared on average at 223.8±186.34 days (rang, 14–505 days). Among the remaining 8 patients, the pleural cavity diminished on average 197.4±149.31 days (range, 26-437 days) after the treatment (Figure 3).
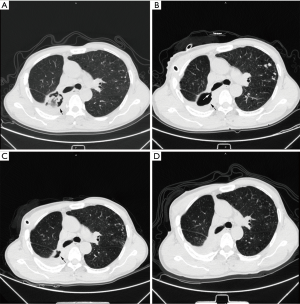
Adjuvant treatment of primary disease
For the 10 cancer patients, cons and pros of chemotherapies and BPF treatment were meticulously balanced. While one developed BPF after finishing all 4 cycles of chemotherapies, and 2 were in stage Ia; only 7/10 patients were in prominent conflicts of treatments: 3 BPFs that occurred prior to chemotherapies completed 4 cycles without disruption by empyema; the remaining 4 BPFs occurred at the intervals of chemotherapies, two of them postponed chemotherapies due to the presence of pneumonia. The patient with aspergilloma underwent 6 months’ anti-fungus treatment as scheduled. Furthermore, no case in our cohort administrated neoadjuvant therapy.
Follow-up
During follow-up of 50.38±27.84 months (range, 2–111 months), 1 patient died of myocardial infarction at 49 months after treatment of BPF, 1 patient deceased of cancer metastasis at 57 months, and another refused further treatments and was lost to follow after chest tube drainage. The remaining patients were all alive without recurrence (Table 1).
Discussion
BPF is an infrequent but potentially life-threatening complication in lung surgery, with incidences from 1.3% to 34.3% and mortalities from 0.7% to 67% (1-5,7,9). Various risk factors as neoadjuvant therapy and closure of bronchial stump, had been reported (1,10-12). In current study, no case in our cohort administrated neoadjuvant therapy. However, a study performed in our hospital demonstrated that neoadjuvant therapy was independently associated with BPF after pneumonectomy for non-small cell lung cancer (10). Recently reported meta-analysis revealed that both neoadjuvant radiotherapy and chemo-radiation therapy significantly increase the bronchopleural fistula risk but neoadjuvant chemotherapy does not (11). Moreover, in study of Asamura et al. (1), the incidence of BPF after manual suture (4%) was higher than that after stapler suture (1%), but no significant difference was found. Moreover, similar result was also found by Panagopoulos’s research (12).
A bronchial fistula creates a passage between the retaining pleural cavity and patient’s airway conduit. Thus, fluid dissemination to the remnant lungs happens frequently. This is especially dangerous if the remaining pulmonary function is impaired. Therefore, severe hypoxia may occur, especially when extensive flooding happens acutely or when pneumonectomy is performed. Asamura and colleagues (1) reported an overall incidence of BPF in 1.3% (7/533) and 57.1% (4/7) of BPF patients who died of pneumonia and sepsis. In Porhanov and colleagues’ case series (9), 4 patients died from acute pneumonia, sepsis, and ARDS after surgical repair of dehiscence, which led to overall hospital mortality of 8.16% (4/49). Even when the fistula is small and fluid aspiration is minor, refractory empyema and subsequent recurrent pneumonia may aggravate. Symptoms, including fever, purulent expectoration, or even hemoptysis, can be obvious and serious.
An empyema-complicated BPF has been especially difficult to handle despite many treatment options are currently available, including both surgical and bronchoscopic maneuvers. According to the currently available data, surgical closure of the bronchial stump with autologous tissues reinforcement was effective in most, but not all patients. Uramoto and Hanagiri (5) described their 31-years of experience with 19 BPF cases, and reported that repair failed in 11 patients and therefore had a mortality rate at 57.9%. Porhanov and colleagues (9) treated 49 BPF cases via the trans-sternal approach, among which 15 cases underwent bifurcation sleeve resection and 34 had tracheal wedge resections. However, several serious complications occurred (1 right pulmonary artery injury and 7 healing disturbances). Moreover, 6 patients (13.3%) developed delayed healing at the suture site and 2 of them required additional stent placement. The overall mortality rate was 8.16% in Porhanov and colleagues’ study (9), in which 2 patients died from sepsis and respiratory failure due to dehiscence of the anastomosis. Additionally, bronchoscopic technique attempts to spare the fistula, a seemly promising method, also have limitations. The materials included bio-glue, lead shot, polidocanol, silver nitrate, absorbable gelatine sponge, chemical cautery agents, and even valve devices (9,13-15). Glue was only eligible for small fistula, and it was easily spilled over into the pleural space or other bronchi. Varoli and colleagues (7) tried endoscopic multiple polidocanol injection on the edges of the fistula on 35 post-resection BPFs and failed in 34.3% (12/35) cases. Of these 12 patients, 5 died of cardiac or respiratory failure 1–2 weeks after the appearance of the fistula, 1 with a permanent chest drain and 6 with thoracoplasties. In Dutau’s experiences (16), the mortality rate was still high, as 57%, mainly in relationship with overwhelming sepsis and there were 3 (3/7) stent-related complications, including migration and fracture. Furthermore, Leonello Fuso et al. (17) conducted a study comparing conservative treatment and conservative plus endoscopic treatment, which turned out no significant difference of the mean period of time elapsed for the resolution of BPF between two groups. Subsequently, we thought both surgical and bronchoscopic maneuvers were unnecessary for patients who could undergo conservative management.
Even after the fistula has been successfully repaired by surgical or bronchoscopic techniques, fistula-complicated empyema renders an additional barrier before disease cure. Two cases (2/11) in Andreetti’s case series (18) had thoracotomies for the empyema. Massera and colleagues (19) even suggested that open window thoracostomy was possibly the only option for chronic pleural empyema when prolonged chest tube drainage failed to control the infection.
Present study, for the first time, clarified that fistula repair is not necessarily the first step in managing empyema-complicated BPFs. Alternately, infection control and empyema management are the key point of the treatment. Once the pleural cavity is generally clean, formation of granuloma and scar may minimize the cavity and the fistula may occlude gradually. Other thoracic centers have noticed and reported the same phenomenon. Naranjo and colleagues (20) described that 7 post-lobectomy BPF patients were managed with conservative methods, including tube drainage and necessary mechanical ventilation. However, detailed follow-up data concerning the fistulas was lacking and one patient died from BPF-related disease. Presently described conservative methodology certainly has predominant advantages over past experiences and can serve as an alternative to BPF treatment. In detail, the preponderances include: (I) avoid re-operation and corresponding complications; and (II) facilitate easier acceptance of patients and lower cost. Hospitalization was the only cost in patients who underwent our conservative treatment. Although the treatment duration lasted seemingly a long time, the real hospitalized reasons comprised only pneumonia control and cavity lavage associated with shorter hospitalization time. Moreover, surgical repair as open window thoracostomy will take longer duration to achieve completely cure (19). Given that surgery cost was spared and that medicines including antibiotics were similarly applied for both, a conservative method is less expensive than surgery.
Among all procedures, the application of saline irrigation may be argued. Generally, when there is a bronchial fistula, irrigation or lavage may aggregate aspiration pneumonia, which is dangerous. However, based on our experiences, lavage could be safely administered when we set up strict protocols, including indications and manipulations. This maneuver could effectively minimize bacterium count, dilute pleural effusion for easier drainage and promote faster cavity narrowing. To guarantee the safety, the bronchial fistula should be always placed at the highest point of cavity. Irrigation volume and speed are controlled manually with a syringe so that it can be stopped immediately upon cough and possible aspiration. In the present study, there was no lavage-related aspiration or pneumonia. This, as well, explains the safety of subsequent postural drainage. Another doubt might concern the classification of BPF, we demonstrated that air-leaks of included cases were all caused by bronchial stump other than parenchymal, reasons as follows: (I) tiny air leak from parenchymal would not result in diffused pneumonia; and (II) new appeared air-fluid level and residual cavity were all next to the bronchial stump, instead of scattering cavities. Even though fistulas were only confirmed on 4 cases with CT examinations and on 5 individuals with bronchoscopies. The rest patients refused bronchoscopy examination, and fistulas of them were too small to be detected under CT. But above reasons were convincing evidences that the air leak was caused by the bronchial stump.
Conclusions
In conclusion, with experiences from consecutive patient series, present study proved that conservative treatment is both effective and safe for empyema-complicated post-lobectomy BPF cases. For indicated patients, it offers a conservative way of treatment, therefore, it can be advocated over other alternative methods. The consecutive nature of present case series, although involving only 13 cases, strongly suggests the re-productive nature of this therapy.
Acknowledgements
Funding: This work was supported by the projects from Science and Technology Commission of Shanghai Municipality (No.15695840600), the projects from Science and Technology Commission of Shanghai Municipality (13DZ1942805 and 14411962600), Health and Family Planning Commission of Shanghai Municipality (2013ZYJB0003), and Shanghai Hospital Development Center (SHDC12015116).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of Shanghai Pulmonary Hospital approved this study, and consents were obtained from all enrolled patients.
References
- Asamura H, Kondo H, Tsuchiya R. Management of the bronchial stump in pulmonary resections: a review of 533 consecutive recent bronchial closures. Eur J Cardiothorac Surg 2000;17:106-10. [Crossref] [PubMed]
- Høier-Madsen K, Schulze S, Møller Pedersen V, et al. Management of bronchopleural fistula following pneumonectomy. Scand J Thorac Cardiovasc Surg 1984;18:263-6. [Crossref] [PubMed]
- Hollaus PH, Lax F, el-Nashef BB, et al. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. Ann Thorac Surg 1997;63:1391-6; discussion 1396-7. [Crossref] [PubMed]
- Sirbu H, Busch T, Aleksic I, et al. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7:330-6. [PubMed]
- Uramoto H, Hanagiri T. The development of bronchopleural fistula in lung cancer patients after major surgery: 31 years of experience with 19 cases. Anticancer Res 2011;31:619-24. [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18:519-23. [Crossref] [PubMed]
- Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998;65:807-9. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662-7. [Crossref] [PubMed]
- Porhanov V, Poliakov I, Kononenko V, et al. Surgical treatment of 'short stump' bronchial fistula. Eur J Cardiothorac Surg 2000;17:2-7. [Crossref] [PubMed]
- Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. [Crossref] [PubMed]
- Li S, Fan J, Liu J, et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14 912 patients. Jpn J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571-5. [Crossref] [PubMed]
- Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung 2010;188:253-7. [Crossref] [PubMed]
- Fruchter O, Bruckheimer E, Raviv Y, et al. Endobronchial closure of bronchopleural fistulas with Amplatzer vascular plug. Eur J Cardiothorac Surg 2012;41:46-9. [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Dutau H, Breen DP, Gomez C, et al. The integrated place of tracheobronchial stents in the multidisciplinary management of large post-pneumonectomy fistulas: our experience using a novel customised conical self-expandable metallic stent. Eur J Cardiothorac Surg 2011;39:185-9. [Crossref] [PubMed]
- Fuso L, Varone F, Nachira D, et al. Incidence and Management of Post-Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung 2016;194:299-305. [Crossref] [PubMed]
- Andreetti C, D'Andrilli A, Ibrahim M, et al. Submucosal injection of the silver-human albumin complex for the treatment of bronchopleural fistula. Eur J Cardiothorac Surg 2010;37:40-3. [Crossref] [PubMed]
- Massera F, Robustellini M, Della Pona C, et al. Open window thoracostomy for pleural empyema complicating partial lung resection. Ann Thorac Surg 2009;87:869-73. [Crossref] [PubMed]
- Naranjo Gómez JM, Carbajo Carbajo M, et al. Conservative treatment of post-lobectomy bronchopleural fistula. Interact Cardiovasc Thorac Surg 2012;15:152-4. [Crossref] [PubMed]


