|
Original Article
Long-term follow up of patients affected by pulmonary carcinoid at the Istituto Nazionale Tumori of Milan: a retrospective analysis
Pusceddu S1, Catena L1, Valente M1, Buzzoni R1, Formisano B1, Del Vecchio M1, Ducceschi M1, Tavecchio L2, Fabbri A3, Bajetta E1.
1Medical Oncology Unit 2, Fondazione IRCCS "Istituto Nazionale dei Tumori", Milan 20133, Italy. 2Thoracic Surgery Unit, Fondazione IRCCS "Istituto Nazionale dei Tumori", Milan 20133, Italy. 3Pathology Unit, Fondazione IRCCS "Istituto Nazionale dei Tumori", Milan 20133, Italy.
Corresponding Author: Emilio Bajetta, MD, S.C. Oncologia Medica 2, Fondazione IRCCS, Istituto Nazionale dei Tumori, Via Venezian 1, 20133 Milano, Italy. Phone: +39 02 23902500; Fax +39 02 23902149. Email: emilio.bajetta@istitutotumori.mi.it
|
|
Abstract
Neuroendocrine tumors of the lung involve an heterogeneous group of tumors representing a wide range of histological variants, from
well-differentiated typical carcinoid (TC) tumors to poorly differentiated small cell carcinomas. The epidemiology, clinical outcome, and
management of these neoplasms differ significantly from other lung malignancies. The main aim of this report consists in describing the single
Center experience of the Istituto Nazionale Tumori of Milan on neuroendocrine lung tumors, with an emphasis on bronchopulmonary
carcinoid subtypes. From 1986 to 2009, 91 cases of carcinoid tumors were diagnosed; these were divided in two series, according to typical
(66 patients) or atypical ( 25) histotypes. These two groups were compared in relation to various features, including pathologic classification,
clinical behavior, treatment modalities and long-term survival. At the moment of diagnosis 11 patients had locally advanced/metastatic disease,
while 80 patients showed non metastatic disease. The comparative analysis between typical and atypical series disclosed significant
differences in terms of long-term survival; in fact, 5-year and 10-year survival rates were 98 % and 94 % for the first carcinoid series versus
76 % and 18 % for the atypical series, respectively (p<0.001). The median overall survival (OS) was 76 months (range 3-182) for atypical
carcinoids and has not yet been reached for TCs patients. Key words
carcinoid; lung cancer; neuroendocrine; pulmonary
J Thorac Dis 2010;2:16-20. DOI: 10.3978/j.issn.2072-1439.2010.02.01.008
|
|
Introduction
Neuroendocrine (NE) lung tumors originate from a population
of NE cells normally present in the bronchoalveolar structures
and characterized by secretory activity and ability to uptake and
decarboxylate the amine precursors (APUD System cells) ( 1).
These NE phenotypical and morphological characteristics are present
within a broad spectrum of histologies of lung NE tumors,
from relatively indolent typical carcinoids (TC) to histologically
high-grade, biologically aggressive tumors. The spectrum of pulmonary NE tumors, according to the current
WHO classification by Travis, includes four subtypes characterized
by increasing aggressiveness: typical carcinoid (TC), atypical carcinoid
(AC), large-cell neuroendocrine carcinoma and small cell
carcinoma. Among these, TC and AC tumors are considered low-grade and intermediate-grade NE neoplasms, respectively ( 2).
Bronchopulmonary (BP) typical and atypical carcinoid tumors are
uncommon, representing 2% of all pulmonary neoplasms; these
variants are associated with relatively slow growth, and generally
show a favorable outcome ( 3). The annual incidence is approximately
2.3 to 2.8 cases/1 million population ( 4, 5). Most of these
cases consisted in typical carcinoids (80-90%), and occurred more
frequently in the fifth and sixth decades; it's interesting to underline
that they represented the most common lung tumors in childhood.
The female/male ratio is 1.6:1 ( 5). Even if both typical and atypical carcinoids are the expression
of NE lung tumors bearing the best prognosis and outcome, these
two histotypes show some well known differences in terms of histologic
features, molecular biology, pattern of spread and treatment
modalities.
The most important differential criterion between TCs and ACs
is represented by the mitotic count.
The TCs have < 2 mitoses per mm2 in 10 high-power fields
(HPF) without signs of necrosis, while ACs are characterized by 2
to 10 mitoses per mm2/10 HPF and/or foci of necrosis.
In combination with the histologic appearance, the cell proliferation
characterized by low labeling index Ki67 (MIB1) <20%
seems to be the most useful marker to distinguish the low-grade
and the high-grade of malignancy subgroups within the bron-chopulmonary NETs. In particular, the immunoreactivity for nuclear
markers (especially Ki67) is easily accessible and may be helpful
in the differential diagnosis between TC/ACs and small-cell
carcinomas, being high grade NE tumors characterized by a proliferative
cell fraction extremely higher than that of carcinoids (MIB1
>50%) ( 6). Several peptide and amine markers, including Chromogranin
(CgA), neuron-specific enolase (NSE), serotonin, synaptophysin,
and adrenocorticotropic hormone (ACTH), can provide us
with further tools in order to better establish a differential diagnosis
( 6). Molecular genetic changes may be useful as an adjunctive element
to differentiate typical and atypical carcinoids. Retinoblastoma
gene (13q13) and p53 (17p13) mutations, multiple endocrine
neoplasia (MEN) 1 gene activation (11q13), and telomerase activity,
are particularly frequent in atypical carcinoids if compared with
the typical ones ( 7). Although both tumors are malignant, they have a usually benign
behavior, thus being rarely responsible for the death of patients,
even after an extensive period of follow-up. However, ACs are
more aggressive than TCs, metastasizing more commonly both to
regional lymph nodes and distant sites.
Generally, pulmonary TCs appear as central lesions, whereas
ACs are more commonly located peripherally. Bronchopulmonary
carcinoid tumors have a propensity to develop in the right lung
and most of them (90%) are confined to the bronchus ( 8), while the
remaining 10% are located in the lung parenchyma, rarely in the
main carina or trachea. Carcinoids, as other neuroendocrine tumors, may secrete hormone-like substances such as adrenocorticotropic hormone
(ACTH) and arginine vasopressin, thus causing paraneoplastic syndromes.
The classic carcinoid syndrome is very rare in patients
with pulmonary carcinoids (+/- 2%) ( 9). Presenting symptoms in
both the subtypes can be cough, hemoptysis, or signs of bronchial
obstruction; in some cases, the patients can be asymptomatic. The treatment of choice is surgery for localized disease. In advanced
or metastatic disease no effective medical treatment exists
( 10, 11). The aim of this retrospective analysis is to review low-grade lung NETs treated at our institution in order to assess
any correlation between the histological subtypes and clinical behavior,
treatment response and long-term survival.
|
|
Patients and methods
We retrospectively reviewed a cohort of 91 consecutive patients
affected by bronchopulmonary carcinoids referred at the Medical
Oncology Unit 2, National Cancer Institute of Milan, during a
23-year period from 1986 to 2009.
The analyzed data included patients' age and sex, presenting
symptoms, stage at diagnosis, histophatological findings, presence
or absence of carcinoid syndrome, In-111-labeled OctreoScan
(scintigraphy with radiolabelled octreotide), treatment modalities
and overall survival (OS). The histological subtypes were classified
according to the 1999-2004 Travis-WHO classification criteria by
pathologists from our Institution ( 2). For staging and follow-up we used brain, chest and abdominal
computed tomography scan (whole body CTscan). In case of suspected
relapse, we have also performed Octreoscan to integrate CT
over the last 15 years in order to better define the staging at diagnosis
and the consequent treatment plan. Fibrobronchoscopy was performed
in those patients who had endobronchial carcinoids or in
case of suspicion of bronchial recurrence. The Kaplan-Meier product
limit estimator was used to graphically display the survival
curves, with death from any cause as outcome; Mantel-Cox
log-rank test was used to compare survival between different
groups. A p value of <0.05 was considered statistically significant.
|
|
Results
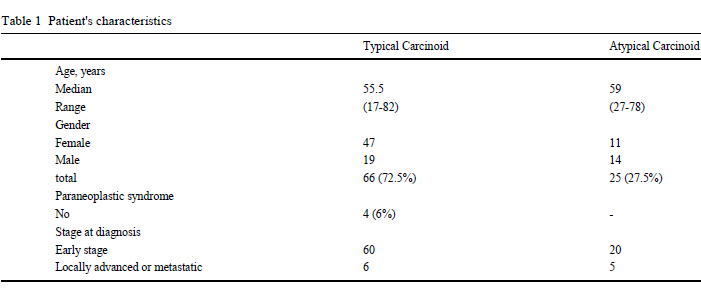
The patients' baseline characteristics are summarized in Table 1.
The cohort consisted of 33 men and 58 women, with a median age
of 56 years (range 17-82), affected by typical carcinoids (66 cases)
and atypical carcinoids (25 cases). Some patients showed accom
panying pathologies including hypertension (25 patients), diabetes
mellitus (10 patients) and cardiovascular diseases (4 patients). The majority of patients are asymptomatic, and, in the remaining patients,
the most notable presenting signs/symptoms were invariably
respiratory consisting in hemoptysis, cough, ronchi, and dyspnea
other than weight loss. According to the natural history of these
malignancies, only 4 patients had carcinoid syndrome with facial
flushing and diarrhea as the commonest symptoms. Octreoscan and
positron emission tomography (PET) scan were performed at diagnosis
in 49 patients as staging procedures, providing us with an important
help for the detection of primary tumor or disease recurrence.
|
|
Treatment
Of the 91 patients reviewed at our Institute, 80 (88%) were classified
in early stage at diagnosis, 60 of whom (75%) were classified
as CTs and 20 (25%) as ACs, respectively.
All patients underwent radical surgery and no adjuvant treatment
was performed after surgery.
After surgical treatment, 24 patients (30%) developed a recurrent
tumor (11/60 CTs and 13/20 ACs), being liver the most frequent
site of distant relapse in 67% of all the histotypes. Other sites
of relapse included lung (37%), lymph nodes (33%) and bone
(17%) with a similar distribution between TCs and ACs. Locally
advanced disease and distant metastases were observed in 11(12%)
patients (6 TCs and 5 ACs); the majority of these patients had two
or more sites of distant metastases, being liver and lung the most
frequent targets.
Nevertheless, in this prolonged period of observation of such retrospective
study, all patients with relapsed, advanced or metastatic
disease were suitable for medical treatment. In these series of patients,
23 were treated with somatostatin analogs, 10 with 5-FU or
CDDP-based chemotherapy.
|
|
Survival analysis
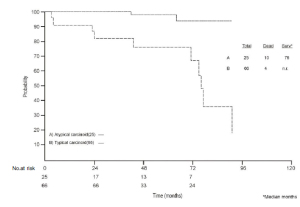
The median overall survival (OS) calculated for ACs was 76
months (range 3-182) while, up to date, for TC patients the median OS has not yet been reached ( Fig 1). Five and ten-year survival rate were 98 and 94% for TCs and 76
and 18% for AC, respectively (p < 0.001).
|
|
Discussion
Pulmonary carcinoids are rare (accounting for less than 2% of
all bronchial tumors) ( 3), and they represent a spectrum of neuro-
endocrine tumors with different behaviors and prognoses depending
on histopathological features and differentiation. We reviewed
retrospectively the histology of our bronchopulmonary carcinoid
tumor series using the 1999 Travis classification with the
aim to gather information about clinical outcomes and survival related
to histopathological subtype and stage. Our data on the demographic characteristics demonstrated a different
gender distribution in both typical and atypical histotypes.
The female/male ratio is 2.5:1 and 1:1.5 in TC and AC, respectively;
then, according to literature, the patients with atypical carcinoids
were generally older at presentation than those bearing typical
subtype (median 59 vs 55 years, respectively). These findings,
although with limited numbers of patients, confirm the relationship
histological switching from low- to high grade, tumor aggressiveness,
increasing age and male incidence for these tumor types,
as postulated by other authors ( 4, 12); in fact, we confirm the hypothesis
that male sex and older age can be a negative prognostic
factors in BP carcinoid tumors. Typical carcinoids excellent long-term overall survival rate,
while atypical carcinoids showed worse survival rate, thus the aggressiveness
of this form and its disappointed prognosis especially
in advanced stages. The 5-year survival rate for neuroendocrine tumors
of the lung rang from 87 to 97% for TCs and from 56 to 77%
for ACs ( 12-14). The very favorable prognosis for TC is justified
by the low percentage of distant metastases after radical surgery
(5-20%); on the contrary, 30-70% of individuals with ACs exhibit
regional lymph node or distant metastatic disease ( 15). In fact, Fink
et al. reported that TC patients had N1 disease in10% of cases and
N2 in 3% (no N3 observed), while AC patients had N1 in 29% of
cases, N2 in 14% and N3 in 14%. Distant metastases have usually
been observed at the liver, skeleton, central nervous system, skin
and mammary glands (3% for TC and 21% for AC) ( 5). AC tumors have demonstrated in most of the studies a poorer
prognosis if compared to TC,due to a major biological aggressiveness;
we have confirmed these characteristics also in our experience,
where the comparative analysis between the two subtypes
disclosed statistically significant difference in overall survival ( Figure I). TCs showed a significant better survival than ACs
(5-year-survival rate 98% and 76%, respectively), according to the
range of the rates reported previously, with a lower percentage of
recurrence of TC (18%), in comparison to AC (65%), after surgery
in the early stage. The results of this retrospective analysis also confirm that
Travis classification is fundamental in the clinical management of carcinoid tumors in order to determine the prognosis of both typical
and atypical subtypes. Consequently, these data support the fact
that accuracy of the pathological diagnosis and clinical staging are
essential to decide the appropriate management of such disease.
When the tumor is accessible (35-70% of BP carcinoids), fibrobroncoscopy
remains the most important tool for diagnosing BP
carcinoids, while the fine-needle aspiration through computed tomography
scan (CTscan), is preferred for peripheral lesions ( 7).
Mediastinoscopy, video-assisted thoracic surgery, and thoracotomy
are other alternatives in case of not feasibility of less invasive diagnostic
procedures. CT scan is recommended to define the clinical stage (TNM) at
diagnosis and during the follow up, and newer modalities of
scintigraphy using radiolabelled somatostatin analogs, such as Octreoscan
or (68) Ga-DOTATOC PET, are useful in case of doubts
regarding the nodal status, the tumor diffusion and the identification
of an unknown primary tumor site. Regarding the biochemical
markers, measurement of urine 5-hydroxyindole-3acetic (5-HIAA)
has an 88% specificity for the detection of 5-HT producing bronchopulmonary
NE tumors. CgA is a sensitive marker of NETs,
even if it can be normal in carcinoids until metastatic disease develops
( 16). When feasible, radical surgery offers the only chance of cure,
representing the treatment of choice for localized disease ( 10). The
medical treatment of metastatic disease includes biotherapy with
somatostatin analogs, possibly associated with interferon α-2a,
and chemotherapy. Somatostatin analogs can provide good symptomatic
relief, prolonging the time to progression in low-grade histotypes;
however, most of the published data refer to small numbers
of patients, thus no statistically significant conclusions can be
taken out ( 11). The use of various chemotherapeutic agents (doxorubicin,
5-fluorouracil, dacarbazine, cisplatin, etoposide, streptozotocin
and carboplatin), can produce not durable objective responses
or stabilizations of disease ( 7). More promising results, especially
ACs, have been reported using targeted radiotherapy with
111In octreotide and 131I meta-iodo-benzyl-guanidine (MIBG)
( 13). Liver embolization with gel foam has been used in few patients
with symptomatic disease and predominant tumor burden localized
at liver. Transient stabilizations and objective responses
have been occasionally observed ( 17). Generally, in patients affected
by TCs or ACs bearing an indolent course, octreoscan positivity
and/or carcinoid syndrome, our first-line treatment consists in somatostatin
analogs (LAR octreotide/lanreotide). Following the failure
of this therapeutic regimen, we have switched to 5 fluorouracil
(5FU)-based chemotherapy, or polychemotherapy including the
combination of 5-fluorouracil, dacarbazine , and epirubicin (FDE
regimen, 5FU + Dacarbazine + Epirubicine), or capecitabine plus
oxaliplatin (the XELOX regimen), treatment schemes that had
demonstrated activity in BP carcinoids in our previous clinical trials
( 18-23). Patients having shown progressive disease during therapy
based on these regimens were treated with either cisplatin plus
etoposide or metabolic radiotherapy. Liver chemo-embolization was used as second or third line treatment when most of the tumor
burden was located at liver.
|
|
Conclusion
Many questions remain to be answered regarding the knowledge
of biological mechanisms of BP neuroendocrine system and
concerning the optimal treatment of typical and atypical carcinoid
tumors. In particular, what should be the best surgical strategy for
correct staging in carcinoid tumors (adequate lung resection with
or without complete mediastinal nodal dissection) and the role of
adjuvant chemotherapy or mediastinal radiotherapy in AC tumors
with lymph node involvement are still under debate. Regarding the
medical treatment of metastatic disease, further studies are necessary
to clarify histology-specific mortality and tumor treatment sensitivity,
also considering genetic characteristics and other biological
aspects. Traditionally, cytotoxic agents are of limited efficacy
in the treatment of neuroendocrine tumors. Recent investigations
have contributed to identify a number of biological features in this
family of neoplasms that could represent targets for tailored treatment.
NETs seem to have an extraordinary tumor vascularization,
as documented by high expression of proangiogenic molecules
such as vascular endothelial growth factor, overexpression of certain
tyrosine kinase receptors such as EGFR, IGFR and by activation
of the downstream signaling pathway (PI3K-AKT-mTOR).
Various clinical trials for patients with carcinoids are currently recruiting
patients. The main objective consists in setting up a precise
targeted therapeutic strategy according to the biologic and genetic
advances.
|
|
Acknowledgements
The Authors would like to thank the scientific service of the Italian
Trials in Medical Oncology (I.T.M.O. Group) and Fondazione
Giacinto Facchetti per lo Studio e la Cura dei Tumori O.N.
L.U.S. for the editorial assistance.
|
|
References
- Pearse A. The cytochemistry and ultrastructure of polypeptide hormone producing cells of APUD series and the embryologic, physiologic and pathologic implications of the concept. J histochem Cytochem 1969;17:303-13.
[LinkOut]
- Travis WD, Corrin B, Shimosato Y, Brambilla E. The histological typing of lung and pleural tumors. In: WHO/IASLC Classification of Lung and Pleural Tumors.3rd ed. Berlin: Springer; 1999.
- Chugta T, Morin J, Sheiner N, Wilson J, Mulder D. Bronchial carcinoid: twenty years experience defines selective surgical approach. Surgery 1997;122:801-8.
[LinkOut]
- Gatta G, Ciccolallo L, Kunkler I, Capocaccia R, Berrino F, Coleman MP, et al. Survival from rare cancer in adults: a population-based study. Lancet Oncol. 2006;7:132-40.
[LinkOut]
- Fink G, Krelbaum T, Yellin A, Bendayan D, Saute M, Glazer M, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51.
[LinkOut]
- Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005;29:179-87.
[LinkOut]
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21.
[LinkOut]
- Cooper WA, Thourani VH, Gal AA, Lee RB, Mansour KA, Miller JI. The surgical spectrum of pulmonary neuroendocrine neoplasms. Chest 2001;119:14-8.
[LinkOut]
- Hage R, de la Riviè re AB, Seldenrijk CA, van den Bosch JM. Update in pulmonary carcinoid tumors: a review article. Ann Surg Oncol 2003;10:697-704.
[LinkOut]
- Kosmidis PA. Treatment of carcinoid of the lung. Curr Opin Oncol 2004;16:146-9.
[LinkOut]
- Katai M, Sakurai A, Inaba H, Ikeo Y, Yamauchi K, Hashizume K. Octreotide as a rapid and effective painkiller for metastatic carcinoid tumor. Endocr J 2005;52:277-80.
[LinkOut]
- Garcia-Yuste M, Molins L, Matilla JM, Gonzalez-Aragoneses F, Lopez-Pujol J, Ramos G, et al. Trends in prognostic factors for neuroendocrine lung tumors:analys of the experience of the Spanish multicentre study of neurendocrine tumours of the lung. Arch Bronconeumol 2007;43:549-56.
- FilossoPL, Rena O, Donati G. Bronchial carcinoid tumors: surgical management and long-term out come. J Thorac Cardiovasc Surg 2002;123:303-9.
[LinkOut]
- Travis WD, Rush W, Flieder DB, Falk R, Fleming MV, Gal AA, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol 1998;22:934-44.
[LinkOut]
- Beasley MB, Thunnissen FB, Brambilla E, Hasleton P, Steele R, Hammar SP, et al. Pulmonary atypical carcinoid: predictors of survival in 106 cases. Hum Pathol 2000;31:1255-65.
[LinkOut]
- Zuetenhorst JM, Korse CM, Bonfrer JM, Peter E, Lamers CB, Taal BG. Daily cyclic changes in the urinary excretion of 5-hydroxyindoleacetic acid in patients with carcinoid tumors. Clin Chem 2004;50:1634-9.
[LinkOut]
- Granberg D, Eriksson B, Wilander E, Grimfjard P, Fjallskog ML, Oberg K, et al. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann Oncol 2001;12:1383-91.
[LinkOut]
- Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L, et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol 2007;59:637-42.
[LinkOut]
- Bajetta E, Rimassa L, Carnaghi C, Seregni E, Ferrari L, Di Bartolomeo M, et al. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer 1998;83:372-8.
LinkOut]
- Ferrari L, Della Torre S, Collini P, Martinetti A, Procopio G, De Dosso S, et al. Kit protein (CD117) and proliferation index (Ki-67) evaluation in well and poorly differentiated neuroendocrine tumors. Tumori 2006;92:531-5.
[LinkOut]
- De Dosso S, Bajetta E, Procopio G, Cortinovis D, Buzzoni R, Catena L, et al. Pulmonary carcinoid tumours: indolent but not benign. Oncology 2007;73:162-8.
[LinkOut]
- Bajetta E, Procopio G, Pusceddu S, Pietrantonio F, Milione M, Maccauro M, et al. From biology to clinical experience: evolution in the knowledge of neuroendocrine tumours. Oncol Rev 2009;3:79-87.
[LinkOut]
- Bajetta E, Ferrari L, Procopio G, Catena L, Ferrario E, Martinetti A, et al. Efficacy of a chemotherapy combination for the treatment of metastatic neuroendocrine tumours. Ann Oncol 2002;13:614-21.
[LinkOut]
Cite this article as: Pusceddu S, Catena L, Valente M, Buzzoni R, Formisano B, Del Vecchio M, Ducceschi M, Tavecchio L, Fabbri A, Bajetta E. Long-term follow up of patients affected by pulmonary carcinoid at the Istituto Nazionale Tumori of Milan: a retrospective analysis. J Thorac Dis 2010;2:16-20. doi: 10.3978/j.issn.2072-1439.2010.02.01.008
|