Clinical application of near-infrared thoracoscope with indocyanine green in video-assisted thoracoscopic bullectomy
Introduction
Bullectomy under video-assisted thoracoscopic surgery (VATS) has been considered as the preferred intervention in most patients with spontaneous pneumothorax. The advantages of VATS bullectomy in blood loss, perioperative pain and hospital stay when comparing to open surgery are well accepted (1,2). However, some researches, including one important meta-analysis (3), have indicated a significant weakness of minimally invasive procedure, the higher recurrence rate. The postoperative recurrence is partly attributed to the remains of bullous lesions, particularly, in cases of which the boundaries of the lesions are difficult to identify.
The application of indocyanine green (ICG) and infrared thoracoscope prototype in detecting pulmonary bullae was first reported by Gotoh et al. (4). However, the high dose of ICG with short observation time made their method difficult to be applied in clinical practice. In this pilot study, we used the state-of-the-art near-infrared (NIR) thoracoscope and tried to set up an optimal ICG dosage.
Methods
The PINPOINT NIR thoracoscope system (Novadaq Technologies Inc., Ontario, Canada) was used for operation. The optical system can transmit both visible light and 808±5 nm NIR laser. Pulse duration is 17 ms in the maximum setting at 20 pulses per second. Tissue is illuminated continuously with fluorescent excitation light and periodically with visible light. Fluorescent images are acquired during a time period when only the excitation light is supplied as illumination, while color images are acquired during a time period when the combination of both excitation light and visible light are supplied as illumination (Figure 1).
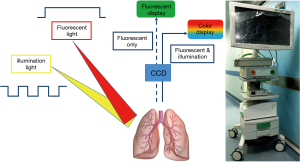
Two different modes for demonstration are used intra-operatively. In fluorescent mode, the emitting density of NIR light from ICG molecule is captured by the NIR charge coupled device (CCD) matrix and display as black and white signal on the monitor screen. In syncretic mode, further after effects are utilized. First, the original density of NIR signal is interpreted according to green color gradation. Then, the images from NIR CCD and white light CCD are synchronized and fused into one image, so that the two different spectrums can be observed simultaneously.
Patients with spontaneous pneumothorax and poorly identified bullae intraoperatively both under normal light and with air recruitment were included. Eventually, two male patients with spontaneous pneumothorax were enrolled. Both patients were confirmed to have no drug allergy to iodine or ICG. The mean age of the patients was 25.5 years (mean, 22–29 years). The study was approved by the Institutional Review Board of Peking University People’s Hospital (IRB No. 2015PHB157-01) and registered at ClinicalTrials.gov (ID: NCT02611245). Written informed consent was signed by both patients.
After general anesthesia, VATS bullectomy was performed. Both patients underwent thoracoscopic explorations under normal white light and then with air recruitment before ICG injection. NIR mode was used to observe the fluorescent status of the lung after the injection of different doses of ICG. Following procedures were operated under syncretic mode. Surgical specimens were submitted for pathologic examination (Figure 2).

Image analysis software (ImageJ v1.44) was used to quantify the fluorescent density. A background reading was taken from adjacent normal lung tissue in order to generate a signal to background ratio (SBR). The SBRs were assessed by an average of 3 measurements of the fluorescent density.
Results
No perioperative complications were observed. The fluorescent signal was detected in normal lung tissue 10.5 seconds (mean, 10–11 seconds) after the ICG bolus, and lasted up to 525 seconds (mean, 480–570 seconds) (Table 1). The fluorescent densities were measured in the second patient in the sampling area of fluorescent image, showed that the fluorescent densities of bullous lesions were significantly lower than adjacent normal lung tissue (Figure 3).

Full table

The bullae of both patients were difficult to identify under surgeon’s inspection in visible light (Figure 4). In the first patient, the ICG dose was 0.2 mg/kg, the boundaries of bullae were visible in fluorescent mode. However, in syncretic mode, the signal was not strong enough to create visible pseudo green color according to surgeon’s view (Figure 4). In the second patient, the ICG dose of 0.6 mg/kg was able to precisely demonstrate the boundaries of bullae in both modes (Figures 2,4). The max SBR between normal lung tissue and bullous lesions in 0.2 and 0.6 mg/kg ICG group were 5.77 and 6.32, respectively.
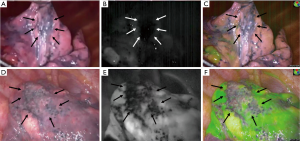
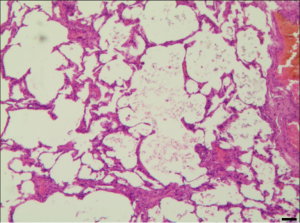
All the resected lesions were proved bullous changes, emphysematous changes, or both microscopically (Figure 5).
Discussion
ICG is a tricarbocyanine fluorescent dye which was proved safe for intravenous injection for human for almost 60 years (6). It bounds rapidly to serum protein once injected and is distributed throughout the body with blood flow. Eventually, ICG molecules are absorbed and secreted by liver cells. The initial application of ICG is to evaluate liver function and cardiac output (6). However, its utilization in thoracic surgery is still preliminary because the detection system has not been developed into clinical size until recently. Some reports have applied NIR thoracoscopy with ICG to identify segmental fissure in video-assisted thoracoscopic segmentectomy (7) and to evaluate the blood supply of gastric conduit in robot-assisted minimally invasive Ivor Lewis esophagectomy (8). In order to get the fluorescent imaging in this study, we illuminate the tissue of interest with light at the excitation wavelength (about 750 to 800 nm) while observing it at longer emission wavelengths (over 800 nm).
In VATS bullectomy, one major difficulty is to locate and identify the boundaries of the bullous lesions, which is mostly attributed to comprehensive palpation and inspection of the lungs. This study indicates that fluorescent-guided surgery is a useful solution to this problem. Theoretically, bullous or emphysematous lesions have lower tissue density and decreased blood flow than adjacent normal lung tissue, thus lower concentration of ICG molecules are distributed. The sudden drop of ICG concentration can be real-timely identified by NIR thoracoscope, and the border of the lesion is clearly demonstrated.
As for the dosage of ICG, Matsumoto et al. used 0.5 mg/kg ICG to delineate the border of a localized emphysema using the PDE system (9). Kasai et al. also used 0.5 mg/kg ICG to identify lung intersegmental borders (7). Oh et al. compared ICG concentrations of 0.3, 0.6, and 3.0 mg/kg in rabbits, and found that 0.6 mg/kg of ICG was an optimal dosage with fluorescent intensity and relatively long washout time (10). In this study, we compared two different ICG doses. The lower dose of 0.2 mg/kg was insufficient for syncretic mode while merely visible in fluorescent mode (Figure 4). We therefore recommend the higher dose of 0.6 mg/kg, which was sufficient for both modes, and was more convenient in clinical practice. The possible observation time under fluorescent light was 520 s (mean, 480–570 s), which was enough for surgeon to explore the thoracic cavity and resect the lesion.
Gotoh et al. first reported the clinical application of infrared thoracoscope prototype in VATS bullectomy in 2007 (4). However, their method needed 3 mg/kg ICG while the possible observation time had only 150–210 s (4). The high dose of ICG with short observation time made that method difficult to be applied in clinical practice. In contrast, our method needed only 0.6 mg/kg ICG while the possible observation time was 525 s averagely.
For patients with clear view of bullae in VATS bullectomy, fluorescent system is not mandatory. However, in cases that is lack of visible bullae, the NIR-guided surgery technique shows great potential. Further application of this system include localized emphysema or infected lesions, in which the border between lesion and normal tissue can be precisely identified (9).
There were several limitations in this study. First, this was a pilot study with only two patients included. Preliminary data could only indicate the potential of this technique. Some tissues, like the fibrous tissue, which have decreased blood flow may present as low fluorescent signal as bullous tissues, so it would need more studies to apply this technology into real clinical practice. In addition, more ICG doses should be tested before the optimal dose was defined.
In conclusion, NIR thoracoscopy with intravenous injection of ICG is a safe, accurate and real-time method to detect bullous lesions of lung tissue in human subjects, especially in patients whose bullous lesions difficult to be found under normal light. This newly developed technology will be used widely in VATS bullectomy in the future.
Acknowledgements
Peking University People’s Hospital Research and Development Foundation (NO. RDC2015-33) provided all funding for this study.
Footnote
Conflicts of Interest: This manuscript was presented as a poster at the 24th Annual Meeting of Asian Society for Cardiovascular and Thoracic Surgery (ASCVTS 2016) at April 06~10, 2016 in Taipei, Taiwan.
Informed Consent: Written informed consent was obtained from the patients for publication of this study and any accompanying images.
References
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [Crossref] [PubMed]
- Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax--long-term results. Eur J Cardiothorac Surg 2011;40:120-3. [Crossref] [PubMed]
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet 2007;370:329-35. [Crossref] [PubMed]
- Gotoh M, Yamamoto Y, Igai H, et al. Clinical application of infrared thoracoscopy to detect bullous or emphysematous lesions of the lung. J Thorac Cardiovasc Surg 2007;134:1498-501. [Crossref] [PubMed]
- Li H, Zhou J, Chi C, et al. Clinical application of near-infrared thoracoscope with indocyanine green in video-assisted thoracoscopic bullectomy. Asvide 2016;3:260.
- Alander JT, Kaartinen I, Laakso A, et al. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int J Biomed Imaging 2012;2012:1-26.
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Matsumoto K, Sano I, Taniguchi H, et al. Thoracoscopic surgery for lung emphysema using an infrared camera. J Cardiothorac Surg 2013;8:134. [Crossref] [PubMed]
- Oh Y, Quan YH, Kim M, et al. Intraoperative fluorescence image-guided pulmonary segmentectomy. J Surg Res 2015;199:287-93. [Crossref] [PubMed]


