Intraoperative frozen sections of the regional lymph nodes contribute to surgical decision-making in non-small cell lung cancer patients
Introduction
Lymphatic metastasis is a significant feature of lung cancer, and lymph node dissection (LND) plays a significant role during lung cancer operations. Presently, systematic mediastinal lymph node dissection (SMLND) or sampling is recommended during radical lung cancer operations (1-3). In 2005, the International Association for the Study of Lung Cancer (IASLC) Staging Committee definited SMLND as the dissection and histological examination of intrapulmonary (lobar, interlobar and segmental) and hilar nodes and, at least, three of the mediastinal nodal stations depending on the lobar location of the primary tumor (4). SMLND not only prolongs the survival of non-small cell lung cancer (NSCLC) patients but facilitates accurate staging, in turn allowing appropriate, multidisciplinary, individualized treatment plans to be formulated (1). Some studies had indicated that survival after complete mediastinal lymphadenectomy tends to improve (5-7). However, the extent to which it improves prognosis, and the role played by SMLND compared to mediastinal lymph node sampling (MLS) in patients with resectable NSCLCs, remain controversial. Although patients may been found to have enlarged (and thus suspicious) lymph nodes during an operation, no study has yet explored whether intraoperative examination of frozen lymph node sections influences surgical decision-making in terms of the extent of pulmonary parenchymal resection and that of LND. In this study, we retrospectively analyzed clinical data on 74 lung cancer patients who underwent intraoperative lymph node biopsies to evaluate the clinical utility of frozen section analysis during pulmonary operations.
Methods
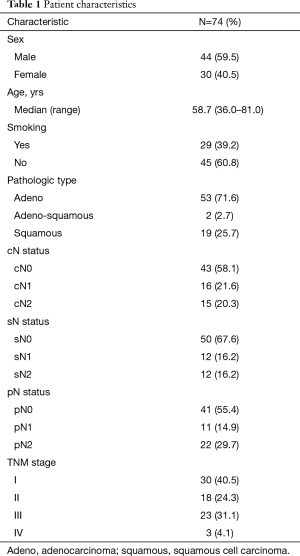
From January 2010 to December 2014, a total of 2,057 consecutive patients who underwent radical lung cancer operations in the Guangdong Lung Cancer Institute were initially enrolled. Ultimately, 74 lung cancer patients who had undergone intraoperative analysis of frozen lymph node sections were identified, and divided by tumor location. Overall, 44 patients were male and 30 were female; all were aged 36–81 years (average: 58.7 years); 29 were smokers and 45 were non-smokers; 47 presented with cough, chest pain, or chest tightness; and 27 were diagnosed upon physical examination, although they lacked any symptoms. The general characteristics of the 74 patients are summarized in Table 1. All underwent computed tomography (CT) or positron emission tomography (PET) for clinical diagnosis of lung cancer. TNM staging used the 7th classification of the IASLC (8). The lymph nodes of the N1 station were considered to be the ipsilateral hilar, interlobar, and lobar lymph nodes, in line with the 2009 IASLC guidelines (9).
Full table
According to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, we defined enlarged lymph nodes as short diameter ≥10 mm preoperatively or we found enlarged lymph nodes in operation based on surgeon’s experience. When preoperative CT or PET showed enlarged lymph nodes, mediastinoscopy or EBUS could be used in the lymph nodes of station 2, 4 and 7. While for the hilar and interlobar lymph nodes, mediastinoscopy or EBUS were limited for accurate staging. Thus, when preoperative CT or PET showed enlarged lymph nodes or we found enlarged lymph nodes of N1 station in surgery, which were suspected metastatic nodes. We suggested to conduct frozen sections of the lymph nodes.
Totally, 26 and 48 patients underwent left lung and right lung cancer surgery, respectively. SMLND was defined as follows: complete resection of stations 1–4 and 7–9 of the mediastinal lymph nodes for right lung cancer, and complete resection of stations 5–9 of the mediastinal lymph nodes for left lung cancer, together with resection of the hilar, lobar, and segmental lymph nodes, and the surrounding adipose tissue (10,11).
Results
Preoperative imaging showed that 30 patients had mediastinal lymph node enlargement; frozen sections revealed metastases in 12 of these patients (40%), including 5 cN1-sN1 patients, 3 cN2-sN2 patients, and 4 cN2-sN1 patients. Eighteen patients had inflamed lymph nodes that lacked metastases. Preoperative imaging showed that 44 patients lacked mediastinal lymph node enlargement; of these, intraoperative analysis of frozen sections of N1 lymph nodes and any suspected metastatic lymph node showed that six patients had metastases (13.6%) (three cN0-sN1 and three cN0-sN2 patients). A significant difference was evident between these two groups (P<0.001); the data are summarized in Table 2. Of the 30 patients with enlarged mediastinal lymph nodes in preoperative imaging, 21 (70%) had lymph node metastases in postoperative pathology analyses. Of the 44 patients who lacked enlarged lymph nodes in preoperative imaging, 12 cases (27.3%) were confirmed to have metastases in postoperative pathology analyses. A significant difference was evident between these two groups (P<0.001).

Full table
In 18 patients for whom frozen section analysis revealed metastatic lymph nodes, postoperative paraffin pathology showed metastases to other lymph nodes in 17 (94.5%). In the other 56 patients, whose frozen sections revealed no metastatic lymph nodes, 15 (26.8%) had metastases to other lymph nodes upon postoperative pathology. Seven (46.7%) of these lacked metastatic N1 lymph nodes but had metastatic N2 lymph nodes. This was partly attributable to skip transfer and occult lymph node metastasis. In seven (46.7%) cases, the intraoperatively sampled N1 lymph nodes were not metastatic; however, postoperative pathology revealed that other N1 lymph nodes were metastatic. Finally, 1 of the 15 cases (6.7%) had intraoperatively negative N2 lymph nodes; however, postoperative pathology revealed that the N1 lymph nodes were metastatic. A total of 41 patients (73.2%) had no lymph node metastases.
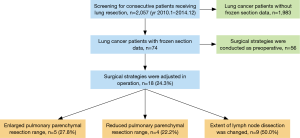
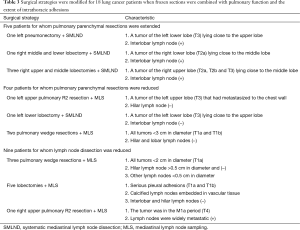
The operative strategies were adjusted intraoperatively in 18 cases when frozen section data were combined with analysis of pulmonary function and intrathoracic adhesions (5 sN-positive, 13 sN-negative cases) (Figure 1). The extents of pulmonary parenchymal resection were extended in five patients (one left pneumonectomy with SMLND, one right middle and lower lobectomy with SMLND, and three right upper and middle lobectomies with SMLND). The extents of pulmonary parenchymal resection were reduced in four patients (R2 resection and MLS in one patient with left upper lung cancer, one left lower lobectomy with SMLND, and two pulmonary wedge resections with MLS). In nine cases, the extent of LND was reduced, including three who underwent pulmonary wedge resection, whose lesions were all <2 cm in diameter, and whose hilar and lobar lymph nodes were not metastatic. We did not perform SMLND in five cases with serious pleural adhesions, whose calcified lymph nodes were embedded in vascular tissue, and for whom intraoperative analysis of the hilar, interlobar, and lobar lymph nodes revealed no metastases. We also did not perform SMLND in one M1a-stage case whose mediastinal lymph nodes were widely metastatic. The surgical strategies are summarized in Table 3.
Full table
Discussion
The optimal resection mode for mediastinal lymph nodes (SMLND or MLS) continues to be controversial (12). In an earlier review, Martini reported that complete SMLND was associated with greater long-term survival (13). Wu et al. conducted a large randomized trial of 532 patients with NSCLC of clinical stage I, II, or IIIA, and found that SMLND significantly improved survival compared to MLS (12). The median survival times were 43 and 32 months, respectively (P<0001). Thus, SMLND has been considered the standard of care for lung cancer resection at most academic centers. The American College of Surgery Oncology Group Z0030 Trial found that SMLND did not improve long-term survival in patients with early-stage (T1 or T2, N0 or nonhilar N1) NSCLC, compared to MLS. In patients with tumors of the right lung, lymph node stations 2R, 4R, 7, and 10R were sampled. In those with tumors of the left lung, stations 5, 6, 7, and 10L were sampled. Any suspicious lymph nodes were also biopsied. If no sampled lymph node showed any evidence of cancer on frozen-section examination, patients were randomized into SMLND or MLS groups. At a median follow-up time of 6.5 years, no survival benefit was evident in the SMLND group (14). Sugi et al. also found no survival difference between SMLND and MLS patients (5-year survival: 81% and 84%, respectively) who had small NSCLCs (<2 cm in diameter) of clinical stage I (15). In our study, 94.5% of patients (17/18) exhibited metastases to other lymph nodes upon postoperative pathology when the frozen sections had revealed metastatic lymph nodes. In the 56 patients for whom frozen section analysis did not reveal any metastatic lymph nodes, only 15 patients (26.8%) had metastases to other lymph nodes confirmed upon postoperative pathology (P<0.001). Thus, if frozen sections are positive, SMLND is necessary. Three patients underwent pulmonary wedge resection and MLS; all lesions were <2 cm in diameter, and the hilar and lobar lymph nodes were free of metastases. SMLND was not performed in five patients with serious pleural adhesions, whose calcified lymph nodes were embedded in vascular tissue, and for whom intraoperative analysis of the hilar, interlobar, and lobar lymph nodes revealed no metastases.
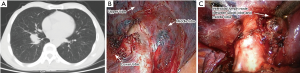
Lobectomy has been considered the gold standard of surgical care for early-stage NSCLC patients, but the extent of parenchymal resection required for local tumor control and the high probability of disease-free survival remains controversial (16-18). Landreneau et al. found that, in patients with small peripheral lung cancers (stage I), anatomical segmentectomy appeared to afford comparable local control, and prolonged disease-free and overall survival. The survival outcomes did not differ significantly from those afforded by lobectomy at a mean follow-up time of 5.4 years (19). Matsuguma et al. found that, in 96 patients with T1N0M0 adenocarcinoma of the lung, those in whom the proportions of ground-glass opacity (GGO) were >50% developed neither lymphatic invasion nor recurrence, and limited surgical intervention was thus advised (20). Dembitzer reported that no significant survival difference was evident between patients treated with lobectomy, or wedge resection or segmentectomy (W/S), after adjustment for tumor size, regardless of the histological subtype or other negative predictors of prognosis (P=0.770). These results suggest that limited resection may be appropriate to treat small tumors, particularly those <2 cm in diameter (21). A meta-analysis also showed that, among stage I NSCLC patients, those who had undergone intentional W/S had survival rates comparable to those who underwent lobectomy (22). In our study, the extents of pulmonary parenchymal resection of five patients were extended intraoperatively to ensure complete (R0) resection; intraoperative frozen sections of lymph nodes were positive in these patients. For example, a 56 years old man was diagnosed of lung cancer of right lower lobe, we planned to perform right lower lobectomy at first, then turned to the right middle and lower lobectomy for frozen section of interlobar lymph node was positive and extracapsular invasive (Figure 2). In addition, based on frozen section data, the extents of pulmonary parenchymal resection of four patients were intraoperatively reduced. Two patients underwent pulmonary wedge resection with MLS; their lesions were small and peripheral, and the lymph nodes were negative.
In recent years, the concept of selective mediastinal lymphadenectomy has attracted increasing attention. LND is performed by reference to the lobe-specific patterns of nodal metastases. Any suspicious lymph nodes are also resected. Selective mediastinal lymphadenectomy can reduce surgery-induced trauma, particularly in elderly patients and those with comorbidities. The survival rate is acceptable, compared to that of early-stage NSCLC patients after complete mediastinal lymphadenectomy (23). Thus, prospective randomized controlled studies are needed to explore the utility of selective mediastinal lymphadenectomy and analysis of intraoperative frozen sections of mediastinal lymph nodes. Such modifications may reduce complications in lung cancer patients and improve their quality of life.
Conclusions
Intraoperative frozen section of regional lymph nodes led to 24.3% operative strategies modification. Given that the aim is complete tumor resection, the extent of resection of the pulmonary parenchyma may be appropriately expanded or narrowed depending on the frozen section, selective LND can also be performed. Such modifications may afford a better quality of life and greater benefits to patients. Examination of lymph nodes frozen sections may be important in surgical decision-making in terms of the extents of both pulmonary parenchymal resection and dissection. These topics require further prospective study.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81001031 and 81372285 to WZ Zhong); the Natural Science Foundation of Guangdong (Grant No. S2013010016354); the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (Grant No. 2012A061400006); the Special Fund for Research in the Public Interest from the National Health and Family Planning Commission of PRC (Grant No. 201402031); and the Research Fund from Guangzhou Science and Technology Bureau (Grant No. 2014Y2-00050).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board and written informed consent was obtained from all patients.
References
- Watanabe S. Lymph node dissection for lung cancer: past, present, and future. Gen Thorac Cardiovasc Surg 2014;62:407-14. [Crossref] [PubMed]
- Keller SM, Adak S, Wagner H, et al. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non-small cell lung cancer. Eastern Cooperative Oncology Group. Ann Thorac Surg 2000;70:358-65; discussion 365-6. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Zhong W, Yang X, Bai J, et al. Complete mediastinal lymphadenectomy: the core component of the multidisciplinary therapy in resectable non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:187-95. [Crossref] [PubMed]
- Massard G, Ducrocq X, Kochetkova EA, et al. Sampling or node dissection for intraoperative staging of lung cancer: a multicentric cross-sectional study. Eur J Cardiothorac Surg 2006;30:164-7. [Crossref] [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]
- Flores RM. Advanced lung cancer: radical surgical therapy. Thorac Surg Clin 2014;24:xiii. [Crossref] [PubMed]
- Wu Yl, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Martini N. Mediastinal lymph node dissection for lung cancer. The Memorial experience. Chest Surg Clin N Am 1995;5:189-203. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. [Crossref] [PubMed]
- Pettiford BL, Schuchert MJ, Santos R, et al. Role of sublobar resection (segmentectomy and wedge resection) in the surgical management of non-small cell lung cancer. Thorac Surg Clin 2007;17:175-90. [Crossref] [PubMed]
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Matsuguma H, Yokoi K, Anraku M, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: A predictor of lymph node metastasis. J Thorac Cardiovasc Surg 2002;124:278-84. [Crossref] [PubMed]
- Dembitzer FR, Flores RM, Parides MK, et al. Impact of histologic subtyping on outcome in lobar vs sublobar resections for lung cancer: a pilot study. Chest 2014;146:175-81. [Crossref] [PubMed]
- Zhang Y, Sun Y, Wang R, et al. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol 2015;111:334-40. [Crossref] [PubMed]
- Jiang W, Chen X, Xi J, et al. Selective mediastinal lymphadenectomy without intraoperative frozen section examinations for clinical stage I non-small-cell lung cancer: retrospective study of 403 cases. World J Surg 2013;37:392-7. [Crossref] [PubMed]


