Bronchial and arterial sleeve resection for centrally-located lung cancers
Introduction
Bronchovascular reconstructive procedures employed in order to avoid pneumonectomy (PN) in functionally unsuitable patients have provided, over time, excellent results, similar or even better than those obtained by PN. In recent years, new successful techniques have been developed that pertain in particular the prevention of major complications and the reconstruction of the pulmonary artery (PA). Encouraging data from increasing number of published experiences support the choice of parenchymal sparing procedures for lung cancer also in patients with good functional reserve. This is even more true if considering trials published in the last 10 years, thus indicating that improved outcome can be achieved with increased experience in reconstructive techniques and perioperative management (1,2). This article discusses the main technical aspects and results of literature.
Indications and preoperative study
The indication for a sleeve resection in patients with lung cancer is a tumor located at the origin of a lobar bronchus, at the origin of the lobar branches of the PA, or both, but not infiltrating the remaining lobes as far as to require PN (Figure 1). In addition, a sleeve resection may be indicated when N1 nodes infiltrate the bronchus and/or the PA from the outside. This condition can frequently be found in patients with left upper lobe tumors needing a combined reconstruction of the bronchus and the PA.
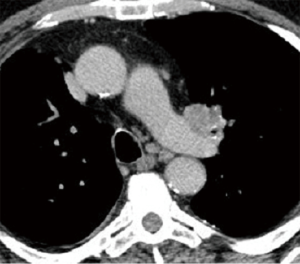
It is not always easy to establish the correct indication for a reconstructive procedure before surgery. Computed tomography (CT) with contrast medium is the most used diagnostic tool. Bronchial infiltration can be confirmed more clearly by a preoperative bronchoscopy, and only in a few cases intraoperative exploration shows a tumor infiltration limited to the external surface of the bronchial wall not visible at the endoscopic evaluation.
In contrast, the correct indication for a sleeve resection of the PA may be more difficult to define by the preoperative study. Angiography and magnetic resonance imaging of the blood vessels can provide useful information to assess the pattern of vascular infiltration, but the decision is usually made during surgery. PA infiltration degree and extension are not always clearly shown at preoperative imaging. Sometimes discrepancies between radiological evidence and intraoperative findings may be responsible for wrong indications, because the preoperative study may overestimate or underestimate the vascular involvement (3).
Establishing the correct indication generally becomes more complex and controversial after induction therapy. At preoperative CT re-evaluation it is usually difficult to distinguish diffuse desmoplastic reaction and fibrosis related to chemotherapy and radiotherapy from residual tumor.
The final decision to perform a sleeve lobectomy (SL) as an alternative to standard lobectomy or PN is therefore generally taken by the surgeon based on intraoperative findings. Because doubts about the presence of viable tumor in the context of fibrotic scarring tissue may persist, extensive use of intraoperative frozen-section analysis is mandatory in order to choose the most appropriate oncologic operation.
Fiberoptic bronchoscopy is the cornerstone of bronchial evaluation. In the setting of a multimodality treatment including surgery it is important that this examination is performed by one of the operating surgeons, ideally with the possibility of comparing the bronchus status after induction therapy with that observed before the induction therapy. Careful evaluation of the bronchial motion can provide useful information on the state of tissues outside the bronchus: stiffness of the bronchial wall may indicate peribronchial tumor infiltration. Endobronchial ultrasonography has improved the accuracy of bronchoscopic evaluation in recent years.
It is still a matter of debate whether, in cases of viable tumor in the main bronchus at presentation that is no longer visible after neoadjuvant therapy, a parenchyma-sparing operation should be considered instead of a PN.
The primary oncologic goal in every case is the complete resection of the tumor with free resection margins. Based on this principle the authors’ approach is to tailor the extent of resection according to the results of frozen-section analysis.
This policy is also justified by the evidence from literature data that PN, especially right PN, is itself a disease, with severe impairment of lung function and quality of life after surgery (4,5). This intervention should therefore be avoided whenever possible.
Another controversial issue is whether the presence of N1 disease should dictate the indication for a PN, because of the higher risk of tumor cell diffusion through the peribronchial lymphatic vessels in the adjacent macroscopically uninvolved lung districts. There is evidence in the literature, for patients with positive hilar nodes, that SL is related to lower morbidity and mortality and better long-term results than PN (6-8). These data support the choice of a parenchyma-sparing operation in this setting.
Technical issues
Dissection
Anatomic and technical considerations in this article mainly refer to upper lobe disease because most surgical procedures are performed for tumors originating in this location, whereas reconstructions for tumors of the lower and middle lobe are rarer.
A crucial step of the operation consists in achieving full control of the proximal portion of the PA. The PA can be isolated on both sides extrapericardially if the infiltration is found only distally near the interlobar fissure. However, in many patients the artery may be involved close to its origin, and therefore the pericardium has to be opened to apply the proximal arterial clamping. The pericardium is generally incised longitudinally behind the phrenic nerve to confirm that the origin of the PA is free from tumor and to allow its preparation. On the right side, extrapericardial preparation can be facilitated with the anterior and medial retraction of the superior vena cava (SVC). When proximal infiltration of the artery is close to the pericardial reflection, the PA can be isolated and clamped transpericardially between the SVC and the ascending aorta. The vessel is then encircled by an umbilical tape. In general, the isolation of the pulmonary veins is less problematic because these vessels are located far from most tumors that are amenable to a sleeve resection. Involvement of the superior pulmonary vein can be found on the left side of tumors infiltrating the anterior aspect of the upper lobe bronchus (which is located closely posterior) or the anterior portion of the fissure.
On the right side the superior pulmonary vein can be involved because of tumors infiltrating the fissural portion of the PA, which is located tightly behind the vein.
Full control of the main bronchus can be achieved without significant problems if a sleeve resection is technically feasible. The core of bronchial and/or PA resection is the dissection in the interlobar fissure. The interlobar fissure is generally approached once complete control of the proximal PA has been achieved. Dissection in this site can be safer after proximal clamping of the artery. Technical expertise and mature surgical judgment are needed, because it is generally in this step of the operation that the tumor can be judged amenable to a sleeve resection or a PN, or considered unresectable. Shrinkage of tumor and fibrosclerotic reaction produced as a consequence of induction therapy usually increase the technical complexity of surgical dissection and may pose doubt in the identification of viable tumor at this site. Frozen-section histology should therefore be performed on all suspicious tissue. However, after chemotherapy or chemoradiotherapy, sleeve resection with reconstructive procedures may also be indicated when indissociable fibrotic tissue with no residual tumor is embedded in the bronchus and/or the PA. In some situations both the upper lobe bronchus and the PA can be encased in fibrotic tissue even without tumor cells at frozen-section analysis. Because lobectomy is technically impossible and PN is the alternative, we think that this is a good indication for a combined bronchovascular reconstruction.
During dissection in the fissural plane, exposure of the arterial branches to the superior segment and to the anterior-basal segment of the lower lobe has to confirm that the vasculature to the lower lobe is free from tumor and can be spared.
On the right side, it is also essential to verify the integrity of the arterial branch to the middle lobe, otherwise the middle lobe should be included in the resection.
We prefer to approach the fissure on the left side starting from its posterosuperior end, at the level of the main PA, proceeding with the dissection anteriorly and inferiorly in a subadventitial plane.
On the right side we expose the intrafissural artery behind the middle lobe and to identify the branch for the superior segment of the lower lobe by dissecting from front to back. To avoid extensive parenchymal dissection in the fissure the exposure can be continued posteriorly at the bifurcation between the upper lobe bronchus and the bronchus intermedius. A crotch lymph node is a frequent finding in this location. If cleavable, this lymph node can be elevated away from the bifurcation, allowing exposure of the PA branch to the superior segment of the lower lobe. Once this branch is identified, the posterior portion of the fissure can be completed with a linear stapler. The bronchus intermedius is encircled just distal to the upper lobe take-off and an umbilical tape is placed to aid the airway division at the appropriate site.
Resection and reconstruction
The resection phase begins once the main and distal PA, the bronchus, and both pulmonary veins have been duly prepared. Before starting the dissection it is useful to clamp the previously prepared main PA. Clamping of the proximal PA is performed after systemic heparinization. In the past we used to clamp the inferior pulmonary vein to obtain backflow control when a sleeve resection of the artery was required. We now prefer to clamp the PA distally to the tumor infiltration. The dose of intravenous heparin represents the only intraoperative management modification adopted by the authors over time. We prefer to administer 1,500 to 2,000 units (about 20–25 units/kg) instead of the dose between 3,000 and 5,000 units that was used in the past. Heparin dose has been reduced to prevent postoperative oozing, especially from the lymphadenectomy sites, and has proved effective in avoiding the risk of thrombosis. Heparin is not reversed by protamin after declamping once the vascular reconstruction has been completed.
Technical aspects of reconstruction of the PA and the bronchus have been addressed in detail in previous publications (9-11) by the current authors and are briefly reported in this article.
Bronchial resection and reconstruction
After complete preparation of the bronchial structures, the mainstem bronchus is divided just proximal to the upper lobe take-off. Once the bronchus has been sectioned proximally (Figure 2), the definitive decision to proceed with a sleeve resection is made based on macroscopic and microscopic findings. The bronchus is divided distally at the inferior upper lobe take-off. Bronchial cuts must be perpendicular to the long axis of the airway. Microscopic tumor found at a bronchial margin requires additional resection of the involved areas or possibly PN.
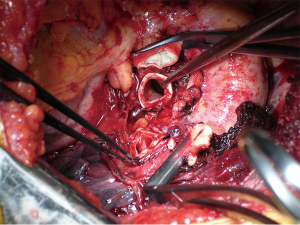
Even though different techniques have been described for bronchial anastomotic reconstruction, the current authors’ preference is for the use of interrupted sutures of 4/0 monofilament adsorbable material (11). Sutures are placed circumferentially starting from the junction between the cartilaginous and the membranous parts of the bronchus on the mediastinal side and proceeding toward the lateral side. Sutures are subsequently tied after their placement has been completed. By accurately calibrating distances between sutures it is possible to compensate for even large-caliber discrepancies between the two bronchial stumps.
This technique prevents torsion of the bronchial axis and gently stretches the circumference of the distal bronchus. The standardized use of continuous running sutures (complete or partial) has also been reported by other investigators (12,13).
Pulmonary artery reconstruction after sleeve resection
End-to-end anastomosis
In patients that require a combined bronchovascular sleeve with PA reconstruction is usually performed after completion of the bronchial anastomosis to minimize the manipulation of the vessel.
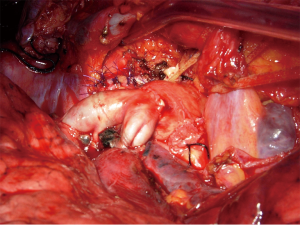
When transecting the artery, both proximally and distally, regular and even margins are desirable, even at the cost of some loss of tissue. This allows proper placement of the stitches and yields an even inside lumen. In addition, regular suture borders facilitate the correction of the large caliber discrepancy that usually occurs. In addition, the exposure of the bronchial stumps is optimal when the artery is divided. If the vascular and bronchial procedures are done simultaneously, the bronchial axis is shortened, and the PA stumps are opposable with acceptable tension. On completion of the bronchial anastomosis, the distance between the two arterial ends will be markedly decreased, and it can be further reduced by elevating the lower lobe while suturing. Restoration of blood flow and removal of the proximal clamp relieves any residual tension. If the distance between the arterial stumps is deemed excessive, the interposition of a prosthetic conduit is indicated. The anastomosis is performed with running 5-0 or 6-0 monofilament non-absorbable material. Additionally, the sutures are placed very carefully to avoid stenosis. End-to-end anastomosis can be technically difficult due to unexpected traction between the stumps and caliber discrepancy (Figure 3).
Long-segment PA reconstruction
In some patients after sleeve resection of the PA an excessive distance between the two vascular stumps may result. This condition could produce a high tension on the anastomosis. Such technical situation may occur, usually on the left side, in those cases requiring resection of a long segment of the PA without associated bronchial sleeve resection, because the lobar bronchus is not involved. In these cases the vascular reconstruction cannot be performed by a direct end-to-end anastomosis and a prosthetic conduit interposition is required.
Although the need for a vascular conduit is not a frequent condition, various materials and different techniques have been proposed for such reconstructive procedure.
Biological materials are generally preferred because of higher biocompatibility and lower risk of thrombosis. The authors have reported the successful use of the autologous and the bovine pericardium (14). More recently, the current authors have introduced the use of porcine pericardium. Intraoperatively, the pericardial leaflet is trimmed to a rectangular shape and wrapped around a chest tube or a syringe of appropriate diameter and sutured longitudinally. In our initial experience this suture was performed manually with a 6-0 monofilament non-absorbable material. More recently we have described a technical alternative with a mechanical suture using a linear stapler for the conduit construction. The creation of a 1–2 cm conduit is so accomplished. When the autologous pericardium is employed the epicardial surface is oriented inside the conduit lumen.
A very interesting alternative for conduit reconstruction is represented by the superior pulmonary vein of the resected upper lobe when the extra-parenchymal portion of this vessel is free from tumor (15,16). The technique used in order to obtain the PV conduit has been described in detail in previous publications (15) (Figure 4).
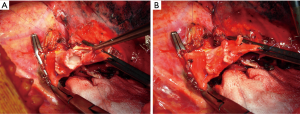
The venous conduit is an ideal substitute for PA replacement since it has adequate thickness and structural similarity with the arterial wall. It is advisable to tailor the length of the biological conduit on the basis of the resected arterial segment, because the elasticity of the two tissues is comparable.
The proximal anastomosis is performed first with running 5-0 monofilament suture. The distal anastomosis is then performed with the same technique, after the conduit length has been checked.
Care must be taken to avoid lengthening of the reconstructed PA, which may cause kinking of the vessel, impaired blood flow and therefore thrombus formation.
For the final success of the reconstruction, it is fundamental to avoid tension on the anastomosis. Tension release can be improved by sectioning the inferior pulmonary ligament and, on the right side, by opening the pericardium around the inferior pulmonary vein.
Viable tissue flap for prevention of bronchoarterial fistula
Bronchoarterial fistula can be effectively prevented by interposing a viable tissue flap between the two structures. The use of mediastinal fat pad, pericardial flap, or pleural flap has been reported (12,17). However, an intercostal muscle flap is preferable because of its excellent vascularization provided by the intercostal artery (18).
The preparation of the flap is performed before opening the chest, and the rib retractor is not inserted until the procedure is completed to avoid crushing the intercostal vessels. The periosteum of the fifth rib is incised and then separated from the bone in continuity with the underlying intercostal muscle. Care must be taken to preserve the muscular insertion to the periosteum to avoid injuring the intercostal neurovascular bundle. The intercostal muscle is then incised in the vicinity of the underlying sixth rib and the anterior insertion of the flap is divided. The pedicle is ligated at its anterior extremity. When the bronchial anastomosis is completed, a large right-angle clamp is slid between the pulmonary artery and the bronchus, and the suture at the extremity of the flap is slid backward around the bronchial anastomosis and between the bronchus and the pulmonary artery. The flap is then twisted until its pleural side is in contact with the bronchial anastomosis and the pleura are secured to the bronchus by interrupted absorbable 4-0 sutures.
Comments
Bronchovascular reconstructive procedures have provided excellent oncological results, similar or even better than those obtained by PN (6-8,19-30) (Table 1). When considering more recent literature experiences, the analysis of long-term survival according to stage and nodal status shows that sleeve lobectomy results in higher survival rates for stages I and II. The survival advantage in stage III patients appears to be limited (6,8,23), and the role of parenchymal sparing operations in patients with N2 disease still remains undefined (21,31).
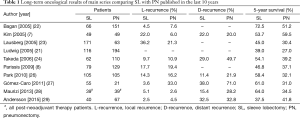
Full table
Nagayasu and colleagues (30) comparing long-term results of bronchovascular reconstructions performed in two different time periods along more than 30 years, reported results dramatically influenced by the high rate of pN2 and even pN3 patients treated during the first period, thus indicating the importance of a correct surgical indication when performing reconstructive procedures for lung cancer.
It has been demonstrated (10,32) that the survival of patients undergoing PA reconstruction is comparable, stage-by-stage, to that reported in the major reviews on lung cancer surgery and bronchial sleeve resection in the literature. The impact of nodal status on survival is also comparable to that reported for bronchial sleeve and standard resection. Once the decision to resect the disease with intent to cure is taken, PA reconstruction can also be proposed as an adequate procedure in this setting. Moreover, there is no significant difference between PA reconstruction alone and PA reconstruction associated with bronchial sleeve in terms of postoperative mortality and morbidity (33). A new interesting information that surfaces from our more recent study (10) and is different from our previous report (32) is that combined bronchovascular reconstructions may offer better survival. This suggests that even this complex lung-sparing operations can be pursued with intent to cure as long as a complete anatomic resection is achieved (10,23,32-36).
However, postoperative morbidity and mortality data reveal overall better results for patients undergoing SL with respect to PN (8,19,20,26) (Table 2).
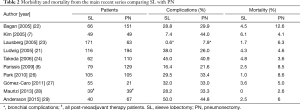
Full table
An interesting meta-analysis (37) including series published between 1996 and 2006 has compared early and long-term outcome of SL with those of PN. A total of 2984 patients have been included in this analysis, of which 21% undergoing SL and 79% undergoing PN. Two-hundred-two patients underwent PA resection and reconstruction in association (164 patients) or not (38 patients) with a bronchial sleeve resection.
Morbidity evaluation from eight studies (7,19-22,24,38,39) showed a pooled incidence of 31.3% in the SL group and of 31.6% in the PN group without statistically significant difference. Similar results were observed limiting the analysis to studies reporting a larger experience (more than 50 patients) of SL. The mean postoperative complication rate reported after PA reconstruction was similar (32.4%) to that reported after bronchial SL and PN. Overall postoperative mortality presented a pooled incidence of 3.5% in the SL group and of 5.7% in the PN group, but this difference did not reach statistical significance. However, when considering only studies with larger number (over 50) of SL, mortality rate was significantly lower in the SL group than in the PN group (6,19-24,40).
Literature data show that PN patients appear to experience a higher rate of cardiac complications when morbidity is evaluated according to the type of complication, while SL patients show increased pulmonary and airway complications incidence (7,19-21,31).
Overall 5-year survival rate from 10 studies (6,7,20-24,36,38,39) was 50.3% after SL and 30.6% after PN, showing a statistically significant difference.
The pooled loco-regional recurrence rate from studies considered in this meta-analysis resulted 16.1% in the SL group and 27.8% in the PN group, but this difference did not reach statistical significance. Otherwise, a significantly lower incidence of local recurrence in favour of SL (SL, 14.5% vs. PN, 28.7%) was reported in the studies with larger number of sleeve procedures (6,20,22,24).
The preservation of lung parenchyma has been indicated by some authors as the possible cause of a theoretical increased risk for loco-regional recurrence after SL. However although in some experiences (31) a higher local recurrence rate is reported for sleeve resection with advanced nodal status (N2), the few studies (7,31) analyzing risk factors for recurrence, show that the tumor stage and the nodal status are the only negative predictive factors, rather than the type of operation performed.
When considering patients with locally advanced non-small cell lung cancer, induction chemotherapy or chemoradiotherapy has become a standardized indication especially in the presence of N2 disease. However, although the beneficial prognostic effects of neoadjuvant therapy have been proved, concern about an increased risk of complications when complex reconstructive procedures are performed after oncologic treatment, has limited the diffusion of such operations within multimodality treatment options. Additional risks may be related to the increased difficulty in surgical dissection caused by diffuse fibrotic reaction, and to the potential healing impairment of the reconstructed bronchus caused by tissue damage and compromised vascularization.
After induction treatment, the dissection of the pulmonary hilum and mediastinum can be difficult and hazardous because the bronchial and vascular structures may be embedded in the desmoplastic reaction and scarring tissue produced by the chemotherapy and radiotherapy. Technical expertise and mature surgical judgment are needed, because it is generally in this step of the operation that the tumor can be judged amenable to a sleeve resection or a PN, or considered unresectable. Shrinkage of tumor and fibrosclerotic reaction produced as a consequence of induction therapy usually increase the technical complexity of surgical dissection and may pose doubt in the identification of viable tumor at this site. Frozen-section histology should therefore be performed on all suspicious tissue. However, after chemotherapy or chemoradiotherapy, sleeve resection with reconstructive procedures may also be indicated when indissociable fibrotic tissue with no residual tumor is embedded in the bronchus and/or the PA. In some situations both the upper lobe bronchus and the PA can be encased in fibrotic tissue even without tumor cells at frozen-section analysis. Because lobectomy is technically impossible and PN is the alternative, we think that this is a good indication for a combined bronchovascular reconstruction. In their initial experience the current authors first reported in 1997 the possibility of performing bronchial and arterial sleeve resection after induction chemotherapy with no mortality, no bronchial and vascular complications, and no local recurrence in the airway. The overall perioperative morbidity rate in a series of 27 patients was similar to that reported in patients undergoing postinduction standard resection in the same period. In addition, 1- and 4-year survival rates (78% and 39%) did not show significant differences from those reported in the standard resection series (65% and 36%) (41,42). These results have subsequently been reproduced and further developed by other investigators worldwide, but available data remain limited, with the largest published series including fewer than 50 patients (43).
In 2013 the authors published the long-term results of their experience comparing SL with PN after induction chemotherapy (28). A total of 39 patients undergoing bronchial and/or vascular reconstruction associated with lobectomy were analyzed and compared with 39 patients undergoing PN over a 14-year period. Postoperative complications occurred in 28.2% of patients receiving bronchovascular reconstruction and in 33.3% of the PN group, without statistically significant difference between the two surgical options. Complications related to the reconstructive procedure occurred in one patient: a late stenosis of the bronchial anastomosis was observed and it was successfully treated by laser and stenting. Postoperative mortality rate in the PN group was 2.6%, while there was no mortality in the SL group. Difference in postoperative mortality was not significant (P=0.3).
The tumor recurrence rate was 20.5% in the SL group (loco-regional in 2 patients, distant in 6) and 30.8% in the PN group (loco-regional in 1 patient, distant in 11), but this difference was not significant. In particular there was no significant difference between the two groups if considering loco-regional recurrence rate only.
Literature data also indicate that lung parenchyma sparing improves postoperative quality of life determining a greater cardio-pulmonary reserve, less pulmonary edema and less right ventricular dysfunction due to a lower pulmonary vascular resistance. In an interesting paper from Ferguson the Quality Adjusted Years Quoted was 4.37 after SL and 2.48 after PN (44). Another paper from Melloul has retrospectively analysed postoperative FEV1 reporting significantly higher values for patients undergoing SL (25). In a prospective study from Martin-Ucar the reported mean FEV1 loss after parenchymal sparing operations was considerably less than after PN, indicating a strongly significant prognostic advantage for patients undergoing SL. Similarly, a recent propensity-matched analysis from Andersson and colleagues (29) compared the outcome after SL with that after PN reported postoperative quality of life results for moving and breathing favouring SL with respect of PN; thus demonstrating the functional benefit after lung sparing reconstructive procedures. If considering SL in comparison with standard upper lobectomy instead of PN, a recent study from the current authors has shown no significant difference in term of functional outcome and quality of life (45). These last evidence enforces the role of SL as an available choice for the treatment of centrally located lung cancers.
Acknowledgements
We wish to thank Dr. Marta Silvi for data management and editorial work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Maurizi G, Rendina EA. Bronchovascular reconstructions for lung cancer: improvements over time. Eur J Cardiothorac Surg 2016;49:306-7. [Crossref] [PubMed]
- Maurizi G, Ibrahim M, Andreetti C, et al. Long-term results after resection of bronchial carcinoid tumour: evaluation of survival and prognostic factors. Interact Cardiovasc Thorac Surg 2014;19:239-44. [Crossref] [PubMed]
- Ciccone AM, D'Andrilli A, Venuta F, et al. Imaging of tumor infiltration of the pulmonary artery amenable to sleeve resection. J Thorac Cardiovasc Surg 2008;136:229-30. [Crossref] [PubMed]
- Venuta F, Anile M, Diso D, et al. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 2007;31:714-7. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1153-61. [Crossref] [PubMed]
- Parissis H, Leotsinidis M, Hughes A, et al. Comparative analysis and outcomes of sleeve resection versus pneumonectomy. Asian Cardiovasc Thorac Ann 2009;17:175-82. [Crossref] [PubMed]
- Rendina EA, Venuta F, Ciriaco P, et al. Bronchovascular sleeve resection. Technique, perioperative management, prevention, and treatment of complications. J Thorac Cardiovasc Surg 1993;106:73-9. [PubMed]
- Venuta F, Ciccone AM, Anile M, et al. Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185-91. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Venuta F, et al. Reconstruction of the bronchus and pulmonary artery. J Thorac Dis 2016;8:S168-80. [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Storelli E, Tutic M, Kestenholz P, et al. Sleeve resections with unprotected bronchial anastomoses are safe even after neoadjuvant therapy. Eur J Cardiothorac Surg 2012;42:77-81. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Reconstruction of the pulmonary artery by a conduit of autologous pericardium. J Thorac Cardiovasc Surg 1995;110:867-8. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Pulmonary artery reconstruction with pulmonary vein conduit for lung cancer: medium-term results. Ann Thorac Surg 2014;98:990-5. [Crossref] [PubMed]
- Cerezo F, Cano JR, Espinosa D, et al. New technique for pulmonary artery reconstruction. Eur J Cardiothorac Surg 2009;36:422-3. [Crossref] [PubMed]
- Tsuchiya R. Bronchoplastic techniques. In: Patterson GA, Deslauriers J, Lerut A, et al., editors. Pearson’s Thoracic and Esophageal Surgery. 2nd ed. Philadelphia, PA: Churchill Livingstone, 2002:1005.
- Rendina EA, Venuta F, Ricci P, et al. Protection and revascularization of bronchial anastomoses by the intercostal pedicle flap. J Thorac Cardiovasc Surg 1994;107:1251-4. [PubMed]
- Gaissert HA, Mathisen DJ, Moncure AC, et al. Survival and function after sleeve lobectomy for lung cancer. J Thorac Cardiovasc Surg 1996;111:948-53. [Crossref] [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Tscholl D, et al. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg 2005;79:1147-52; discussion 1147-52. [Crossref] [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [Crossref] [PubMed]
- Melloul E, Egger B, Krueger T, et al. Mortality, complications and loss of pulmonary function after pneumonectomy vs. sleeve lobectomy in patients younger and older than 70 years. Interact Cardiovasc Thorac Surg 2008;7:986-9. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352-9. [Crossref] [PubMed]
- Maurizi G, D'Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637-43. [Crossref] [PubMed]
- Andersson SE, Rauma VH, Sihvo EI, et al. Bronchial sleeve resection or pneumonectomy for non-small cell lung cancer: a propensity-matched analysis of long-term results, survival and quality of life. J Thorac Dis 2015;7:1742-8. [PubMed]
- Nagayasu T, Yamasaki N, Tsuchiya T, et al. The evolution of bronchoplasty and broncho-angioplasty as treatments for lung cancer: evaluation of 30 years of data from a single institution. Eur J Cardiothorac Surg 2016;49:300-6. [Crossref] [PubMed]
- Fadel E, Yildizeli B, Chapelier AR, et al. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg 2002;74:851-8; discussion 858-9. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Sleeve resection and prosthetic reconstruction of the pulmonary artery for lung cancer. Ann Thorac Surg 1999;68:995-1001; discussion 1001-2. [Crossref] [PubMed]
- Alifano M, Cusumano G, Strano S, et al. Lobectomy with pulmonary artery resection: morbidity, mortality, and long-term survival. J Thorac Cardiovasc Surg 2009;137:1400-5. [Crossref] [PubMed]
- Shrager JB, Lambright ES, McGrath CM, et al. Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann Thorac Surg 2000;70:234-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971-6; discussion 1976-7.
- Nagayasu T, Matsumoto K, Tagawa T, et al. Factors affecting survival after bronchoplasty and broncho-angioplasty for lung cancer: single institutional review of 147 patients. Eur J Cardiothorac Surg 2006;29:585-90. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Yoshino I, Yokoyama H, Yano T, et al. Comparison of the surgical results of lobectomy with bronchoplasty and pneumonectomy for lung cancer. J Surg Oncol 1997;64:32-5. [Crossref] [PubMed]
- Ghiribelli C, Voltolini L, Luzzi L, et al. Survival after bronchoplastic lobectomy for non small cell lung cancer compared with pneumonectomy according to nodal status. J Cardiovasc Surg (Torino) 2002;43:103-8. [PubMed]
- Suen HC, Meyers BF, Guthrie T, et al. Favorable results after sleeve lobectomy or bronchoplasty for bronchial malignancies. Ann Thorac Surg 1999;67:1557-62. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Safety and efficacy of bronchovascular reconstruction after induction chemotherapy for lung cancer. J Thorac Cardiovasc Surg 1997;114:830-5; discussion 835-7. [Crossref] [PubMed]
- Erino AR, Venuta F, De Giacomo T, et al. Sleeve resection after induction therapy. Thorac Surg Clin 2004;14:191-7. vi. [Crossref] [PubMed]
- Bagan P, Berna P, Brian E, et al. Induction chemotherapy before sleeve lobectomy for lung cancer: immediate and long-term results. Ann Thorac Surg 2009;88:1732-5. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Sleeve lobectomy versus standard lobectomy for lung cancer: functional and oncologic evaluation. Ann Thorac Surg 2016;101:1936-42. [Crossref] [PubMed]


