Should tumor with direct adjacent lobe invasion (Tdali) be assigned to T2 or T3 in non-small cell lung cancer: a meta-analysis
Introduction
The significance of tumor with direct adjacent lobe invasion (Tdali) in operable patients with non-small cell lung cancer (NSCLC) is reflected by its incidence of up to 17.1% (1). The appropriate staging of it remains in controversy. In 1999, Japanese Lung Cancer Society assigned invasion of pleural 3 (PL3, parietal pleural and further invasion) to T3, invasion of pleural 1-2 (PL1-2, visceral pleural invasion, VPI) to T2, and Tdali, described as interlobar invasion (ILI) PL3 in the staging system, to T2 too (2). In 2007, the International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project has defined that Tdali should be assigned to T2a unless meeting higher staging criteria (3). Though the staging systems had decided the assignment of Tdali, studies were still reporting inconsistent prognosis of Tdali, either similar to T2 or T3 (1,4-13). Thus, we conducted a meta-analysis to compare the prognosis of Tdali with T2 or T3 disease in patients with NSCLC in order to provide evidence of its staging.
Methods
Search strategy
An electronic search was conducted in Pubmed and Embase using keywords including “lung cancer”, “T2” and “T3”. Searches using these two databases were conducted from their dates of inception to November 20th of 2015 (including articles published ahead of print). Reference list of each included articles were screened for relevance.
Selection criteria
Potentially relevant studies were screened by two independent reviewers (Zhilan Xiao and Jiandong Mei). We included any study that compared the prognosis of Tdali (ILI PL3) to T2 or T3 disease. Cases reports, letters to the editor, correspondence and reviews were excluded. Studies with duplicated data or insufficient comparative data were excluded. Studies that did not mention the T status of Tdali or studies included Tdali of T4 stages were excluded either. Disagreements were resolved by discussion to reach in a consensus and referred to the senior investigators. Data were extracted by Zhilan Xiao and Hu Liao independently and reviewed for consensus.
Statistics
Prognosis of Tdali, Tdali with T2 status and Tdali with T2N0 status was compared with that of T2 or T3 disease respectively. The number of patients analyzed and the number of events in each group were used to calculate the Ln hazard ratio (HR) and its standard error (SE) for each study. Implicit statistical variables were calculated by Tierney’s method using software designed by Matthew Sydes and Jayne Tierney (Medical Research Council Clinical Trials Unit, London, UK) (14). Forrest plots were formulated using RevMan 5.2 (Cochrane collaboration, Oxford, UK) to estimate the prognostic difference of Tdali and T2 or T3. We used a fixed effect model when homogeneity was adequate (P≤0.10, I2≤50%) and a random effect model in other cases (15). An observed HR >1.00 indicated worse outcome for the Tdali group relative to the T2 or T3 group. Moreover, the combined HR would be considered statistically significant if the 95% confidence intervals (CI) did not overlap 1.00. Pearson’s test was used in N stage composition comparison of all three groups. All tests were performed for two sides.
Quality assessment
Quality assessment was performed for each study using the Newcastle-Ottawa scale (NOS) designed for cohort study (16). Comparability of cohorts were designed as whether there was a control for T status of Tdali and whether there was an analysis for N0 subgroup.
Results
Eligible studies
Search in Pubmed and Embase databases yielded 192 and 170 studies, respectively. A summary of the screening, inclusion and exclusion process is presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart in Figure 1.
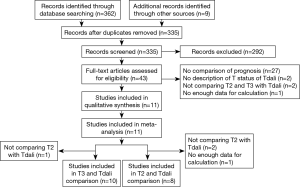
A number of screened studies were written in Japanese with or without an English abstract. A qualified Japanese-Chinese translator was engaged in translation. Difficulties in understanding of the translated text were further discussed with the translator. One study published (17) as meeting abstract was found to be duplicated with another original article (18). One study published as meeting abstract was not included because there were no enough data in the abstract and we failed to contact the authors (19).
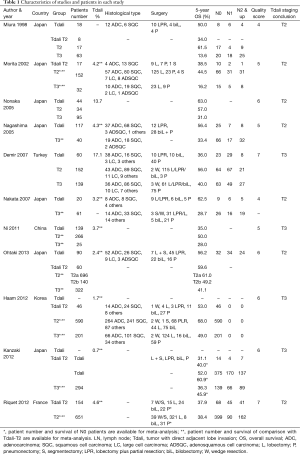
Consequently, comparison between T2 and Tdali included eight studies and comparison between Tdali and T3 included 10 studies. Characteristics of these studies and patients are shown in Table 1. Number of articles and patient number of each cohort were listed in Table 2. Most of the included studies stated that they were retrospective, but some did not mention the study design.
Full table

Full table
It should be acknowledged that the compositions of patients with Tdali in each study were heterogeneous. Some studies included invasion of interlobar pleura or beyond (1,5-8,12,13), whilst others included only invasion beyond interlobar pleura (4,9-11). In regard to the T stage composition of patients with Tdali, some studies limited Tdali to T2 patients (5,7,9-13), whilst others did not (1,4,6), as summarized in Table 1. In most studies, T3 groups included patients with all T3 descriptors, but in Miura’s studies (4), T3 patients with only parietal pleural invasion were included. Similarly, most T2 groups included T2 descriptors, but some studies only included VPI (4,13). As for N stage composition, some studies included various patients with various N stage (4-6,11-13), whilst the others excluded patients with any nodal disease involvement. Some studies also stated that they excluded patients with post-operative death (30 days), patients with neo-adjuvant therapy, patients without incomplete resection of tumor and so on. Most of studies did not state the indicator of surgery. Some of them stated that the indicator was, once with enough reserve, fixed margin was achieved. Haam reported they performed both video assisted thoracoscopic surgery and open thoracotomy (11). The others performed only open thoracotomy or did not mention the type of surgery.
Comparison of overall survival (OS) between Tdali and T2 or T3
Eight and ten studies were included in the meta-analysis for OS of Tdali with unspecific T stage compared with T2 and T3 disease. The comparison between Tdali with unspecific T stage and T2 showed a significant HR [1.39 (1.21, 1.61), P<0.000, I2=45%, fixed effect model, Figure 2A]. In comparison between Tdali with unspecific T stage and T3, the calculated HR was 0.73 (0.57, 0.93) (P=0.01, I2=51%, random effect model, Figure 2B).

Comparison of OS between Tdali-T2 and T2 or T3
Tdali with unspecific T stage constituents T1, T2 and T3 patients. It’s not fair to compare Tdali with unspecific T stage with T2 because Tdali-T3 in the Tdali group would drive the worse prognosis in this group. Thus, we further studied the comparison of OS between Tdali specified in T2 stage and T2 or T3. As some of the studies did not specify its T stage constituent for Tdali or did not provide OS data for Tdali-T2 alone, we excluded these studies from our following comparison. Thus, there were five studies included in comparison between Tdali-T2 and T2 disease and eight studies included in the comparison between Tdali-T2 and T3 disease. The comparison between Tdali-T2 disease and the T2 group showed a significant HR [1.44 (1.23, 1.69), P<0.000, I2=41%, fixed effect model, Figure 2C]. In addition, the HR of patients with Tdali-T2 disease compared to the T3 group was 0.77 (0.64, 0.94) (P=0.008, I2=1%, fixed effect model, Figure 2D).
Comparison of OS between Tdali-T2N0 and T2N0 or T3N0
The N stage constituent of Tdali, T2 and T3 may be different from each other. The potentially higher average N stage of T3 may drive its poor prognosis when compared to Tdali. Thus we further excluded non-N0 patients in order to compare only the prognostic influence of T stage. Four studies entered the comparison between Tdali-T2N0 and T2N0 and three studies entered the comparison between Tdali-T2N0 and T3N0. Comparison between Tdali-T2N0 disease and T2N0 yielded a HR of 1.79 (1.37, 2.34) (P<0.000, I2=0%, fixed effect model, Figure 2E). In comparison between Tdali-T2N0 and T3N0, the HR yielded was 0.98 (0.71, 1.35) (P=0.91, I2=0%, fixed effect model, Figure 2F).
Quality assessment
Studies had an average quality score of 5.55 out of 9. As the number of studies was limited, we did not perform subgroup analysis based on quality score.
N stage comparison
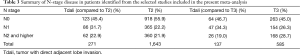
A summary of reported N staging of included studies is presented in Table 3. Studies reporting only N0 patients were excluded in this anaylsis. N stage comparison between T2 and Tdali and between T3 and Tdali were respectively summarized, irrespective of T status of Tdali. Pearson test of number of patients with or without nodal involvement between T2 and Tdali was performed (P=0.001), also for with or without N1 (0.001) and for with or without N2 (P=0.722). Nodal involvement composition between T3 and Tdali was similar (P=0.71), but their N2 composition was different (P=0.021).
Full table
Discussion
The impact of Tdali on staging was first highlighted by Miura and colleagues in 1998 (4). They compared the survival of 18 patients with Tdali to 30 patients with VPI and 63 patients with parietal pleural invasion. They suggested that Tdali, which was similar to VPI, should be categorized as T2 disease. In 1999, a study conducted by Okada comparing prognosis of Tdali with T2 or T3 had the opposite conclusion (20). Further discussion started in 2002 when Morita clearly defined the T stage basis of Tdali as T2 in their research (5). However, their researches did not draw a definitive conclusion. As an important potential independent risk factor, N stage was considered by many researchers as a possible confounding factor. Miura (4), Morita (5) and Nonaka (6) had analyzed the prognosis in N0 cohort and each of their results supported Tdali to be assigned to T2 disease. Further, Morita (5), Nagashima (7) and Nakata (8) had paid attention to the integrity of interlobar pleura. After excluding patients with incomplete interlobar pleura, they agreed on T2. Subsequent to IASLC Lung Cancer Staging Project release new staging system in 2007 (3), several studies failed to achieve the similar conclusion, supporting its inclusion as T3 disease on the contrary (10,11,13).
The present meta-analysis aims to compare the survival outcomes of patients with Tdali to those with T2 or T3 disease. We found that, OS of Tdali, Tdali-T2 and Tdali-T2N0 patients were worse than respective T2 patients. These results suggest that, the malignancy of Tdali has been underrated till now, so similar prognosis of Tdali patients may not be achieved if these patients are treated as T2. In the comparison to T3, Tdali showed better survival both in the whole group analysis and in the Tdali-T2 subgroup analysis. However, survival of Tdali-T2N0 was similar to T3N0 patients. These results suggest that after controlled for T and N stage, prognosis of Tdali is similar to T3.
In Demir, Haam and Riquet’s reports, the tumor size of Tdali was coherently bigger than T2 and smaller than or similar to T3 (1,11,13), though the statistical significance was not mentioned. Tumor size is an important potential independent risk factor, also according to Demir (1). Thus, the difference of tumor size could partially be the reason of difference between Tdali and T2.
Since bigger tumors have more chance to invade adjacent lobe, Tdali-T2N0 group may have more “big tumors” than T2N0. What is important is that, even with some small tumors in Tdali-T2N0 group compared to T3N0, the prognosis of Tdali-T2N0 is still worse than T2N0 but close to T3N0, which suggests that big tumors in Tdali-T2N0 have at least similar prognosis to T3N0 so that they can drive the prognosis of the whole group as poor as T3N0. Thus, these findings suggest that some big Tdali-T2 tumors should be upgraded to T3. What remains unclear is whether the small Tdali-T2 should also be upgraded, and if not, what the cutoff value of the small/big tumor is and how much it should be upgraded.
Similarly, the proposals for the revisions in 8th edition of NSCLC TNM classification suggested that T staging should be upgraded to T3 once tumor size exceeds 5 cm (21). Some big Tdali based on 7th edition will become T3 based on 8th edition of classification. Thus, the remaining question is, based on 8th edition of classification, whether Tdali ≤5 cm still has worse prognosis than T2.
Ohtaki reported that in Tdali with complete interlobar pleura, the prognosis of Tdali-T2a was different from that of T2a (≤5 cm) but similar to T2b (>5 cm) and T3 (>7 cm) based on 7th edition of TNM classification. They concluded that for tumor ≤5 cm (T2a), the T stage should be elevated to T2b once Tdali was detected (10). Ohtaki’s result showed that even if the tumor size is smaller than 5 cm, Tdali still has worse outcome than non-Tdali and should be upgraded in T descriptor. However, our meta-analysis did not perform cancer-specific survival analysis, disease-free survival analysis, analysis for more specific tumor size or analysis for other T descriptor (including visceral pleura invasion, mediastinal pleura invasion and so on) because number of relevant studies was limited. We suggested that Tdali of different size be compared respectively with its non-Tdali counterpart in the future.
Our study suggested that in whole group analysis, the reported nodal involvement was more in Tdali than in T2 (P=0.001) and the difference existed primarily in N1. Though Tdali was not different from T3 in the comparison of all N involvement, more N2 was diagnosed in T3 than in Tdali. N2 in T3 patients may drive bad prognosis of T3 when compared to Tdali. Similarly, nodal involvement of Tdali may drive bad prognosis of Tdali when compared to T2. Thus, it’s crucial to compare Tdali and T2 or T3 within N0 patients to obtain fare comparison. Interestingly, the prognostic difference between Tdali-T2N0 and T3N0 disappeared after controlled for nodal involvement, but T2N0 remains better than Tdali-T2N0. Similar results were yielded in other studies as well. Miura did not reveal any difference between T3 and Tdali in N0, N1 and N2 subgroup analysis respectively (4). Okada (20) conducted Cox proportional hazards regression analysis, which did not show difference between Tdali and T3 irrespective of nodal involvement. However, the similarity of Tdali-T2N0 and T3N0 may also be explained by the limited subjects included in the subgroup meta-analysis. Anyway, we suggest that nodal involvement is an important potential independent risk factor. Thus, more Tdali and T3 cases with specified N stage should be studied.
In 1999, Kamiyoshihara first noticed the influence of interlobar pleural integrity to prognosis (22). Okada (20) mentioned the incompleteness of interlobar pleura should be excluded from Tdali, later agreed by Nagashima and Nakata (7,8). The incomplete interlobar pleura appeared frequently in patients. In 2012, Kanzaki (12) reported 2 incomplete interlobar pleura out of 25 (8.0%) Tdali patients and this number was up to 14 out of 60 (23.3%) in Tdali-T2 and 18 out of 90 (20%) in all Tdali patients in Ohtaki’s study (10). Kamiyoshihara reported up to 74 out of 239 (30.9%) unspecific patients had incomplete interlobar pleura. Ohtaki’s direct comparison between Tdali-A (complete interlobar pleura) and Tdali-D (incomplete interlobar pleura) discovered statistical difference of prognosis of Tdali-A and Tdali-D (5-year OS 52.0% versus 85.7%, P=0.010). The prognosis of Tdali-A is poorer than T2a and similar to T2b and T3. The prognosis of Tdali-D was better than T2a and similar to T1b in his report. Rationally, the prognostic difference of Tdali-A and Tdali-D could be attributed to different lymphatic and vascular invasion in patients. Therefore, it may be important to assess the integrity of interlobar pleura when considering staging and survival of patients with Tdali (18,19).
In terms of surgical treatment, Tdali needs at least lobectomy plus partial resection (LPR), which was performed for 15–55.6% of Tdali patients in the included studies. Most of studies reported that LPR resulted in similar (8,18) or better (1) prognosis than pneumonectomy. LPR was considered to be more appropriate for its better reserve for respiratory and life quality. Right bilobectomy was also suggested to be acceptable in several studies (1,4). Only a few early researchers suggested pneumonectomy for Tdali (23). Local recurrence was uncommon in LPR (4,7,8). Peri-operative death was high in pneumonectomy (1,4). Only a few authors mentioned that lobectomy plus segmentectomy (LPS) was performed for Tdali patients. Anatomic pulmonary resection is generally preferred for patients with T2 disease or higher than wedge resection if patient is eligible for anatomic resection. The invasion in adjacent lobe of Tdali-A may have additional lymphatic and vascular drainage than Tdali-D. With anatomically additional route for metastasis, Tdali-A may require LPS. This may also explain the worse outcome of Tdali-A than Tdali-D when only LPR is performed. In a word, most of current studies supported LPR and bilobectomy as a choice for Tdali. Randomized trials comparing resection techniques may provide more evidence for surgical choice. Segmentectomy should be considered for the invaded lung if possible.
In our analysis, heterogeneity was obvious in comparison between Tdali and T3 (I2>50%). In subgroup analysis of Tdali-T2, the heterogeneity decreased. In N0 subgroup, the heterogeneity was undetectable. Therefore, we believed that the heterogeneity was primarily introduced by different T status of Tdali and the nodal involvement. However, from clinical point of view, heterogeneity could also exist in the tumor size, racial and histological composition, adjuvant therapy, study period, diagnostic techniques, staging criteria changes, the different indications of surgery, the formation of T2 and T3 cohorts and so on.
We did not conduct analysis for publication bias, because in the discussion of staging, whatever the result was, the original article was valuable to be published. And the limited number of the studies also weakened the necessity of the publication bias analysis.
Conclusions
To conclude, our study showed that the prognosis of Tdali is poorer than T2 disease and similar to T3 disease after controlled for T and N status. We suggest that Tdali should be considered to be upgraded to T3 and more evidence is needed to identify which Tdali should be upgraded. Our work challenges the current staging system regarding staging of Tdali, which might be important evidence of future revision of Tdali staging. As the malignancy of Tdali has been underrated till now, more attention needs to be drawn to proper treatment of Tdali patients. In further discussion, reliable evidence should be provided for the staging of Tdali, namely prospectively collected cases with more specific data including T and N status, tumor size, surgery type, integrity of interlobar pleura and other factors that potentially influence the prognosis.
Acknowledgements
Funding: This work was supported by the Key Science and Technology Program of Sichuan Province, China (2013SZ0005 to Lunxu Liu). Lunxu Liu is partially supported by National Natural Science Foundation of China (NSFC81172236 and NSFC81372505) and Key Science and Technology Program of Sichuan Province, China (2013SZ0005). This study was partially supported by Key Science and Technology Program of Sichuan Province, China (2014SZ0148).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Demir A, Gunluoglu MZ, Sansar D, et al. Staging and resection of lung cancer with minimal invasion of the adjacent lobe. Eur J Cardiothorac Surg 2007;32:855-8. [Crossref] [PubMed]
- Japan Lung Cancer Society. General Rules for Clinical and Pathologic Record of Lung Cancer [in Japanese]. 5th ed. Tokyo: Kanahara, 1999.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Miura H, Taira O, Uchida O, et al. Invasion beyond interlobar pleura in non-small cell lung cancer. Chest 1998;114:1301-4. [Crossref] [PubMed]
- Morita R, Kaneko K, Nakamura S, et al. Should interlobar pleural invasion be categorized as T2 or T3. Haigan (Jpn J Lung Cancer) 2002;42:583-7. [Crossref]
- Nonaka M, Kataoka D, Yamamoto S, et al. Outcome following surgery for primary lung cancer with interlobar pleural invasion. Surg Today 2005;35:22-7. [Crossref] [PubMed]
- Nagashima A, Shimokawa H, Takenoyama M, et al. Adjacent Lobe Invasion Beyond the Interlobar Pleura in Non-small Cell Lung Cancer. Haigan (Jpn J Lung Cancer) 2005;45:1-4. [Crossref]
- Nakata S, Sugio K, Sou T, et al. Invasion beyond interlobar pleura in non-small cell lung cancer. The Journal of the Japanese Association for Chest Surgery 2007;21:784-7. [Crossref]
- Ni Z, Jiang G, Ding J, et al. Prognosis and staging of non small cell lung cancer that extends across the fissure into adjacent lobe. Chin J Thorac and Cardiovasc Surg 2011;27:674-7.
- Ohtaki Y, Hishida T, Yoshida J, et al. The clinical outcome of non-small cell lung cancer patients with adjacent lobe invasion: the optimal classification according to the status of the interlobar pleura at the invasion point. Eur J Cardiothorac Surg 2013;43:302-9. [Crossref] [PubMed]
- Haam SJ, Park IK, Paik HC, et al. T-stage of non-small cell lung cancer directly invading an adjacent lobe. Eur J Cardiothorac Surg 2012;42:807-10; discussion 810-1. [Crossref] [PubMed]
- Kanzaki R, Ikeda N, Okura E, et al. Surgical results and staging of non-small cell lung cancer with interlobar pleural invasion. Interact Cardiovasc Thorac Surg 2012;14:739-42. [Crossref] [PubMed]
- Riquet M, Berna P, Arame A, et al. Lung cancer invading the fissure to the adjacent lobe: more a question of spreading mode than a staging problem. Eur J Cardiothorac Surg 2012;41:1047-51. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Margaritora S, Cafarotti S, Lococo F, et al. Surgical treatment in patient with non-small cell lung cancer with fissure involvement: anatomical versus non-anatomical resection. Interact Cardiovasc Thorac Surg 2013;17:S21. [Crossref]
- Leuzzi G, Cesario A, Cafarotti S, et al. Surgical treatment in patient with non-small-cell lung cancer with fissure involvement: anatomical versus nonanatomical resection. J Thorac Oncol 2014;9:97-108. [Crossref] [PubMed]
- Kawachi R, Nakazato Y, Takei H, et al. Should interlobar pleural invasion beyond the lobe be classified as T2 or T3? Interact Cardiovasc Thorac Surg 2012;15:S25.
- Okada M, Tsubota N, Yoshimura M, et al. How should interlobar pleural invasion be classified? Prognosis of resected T3 non-small cell lung cancer. Ann Thorac Surg 1999;68:2049-52. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Kamiyoshihara M, Kawashima O, Sakata S, et al. Does an incomplete interlobar fissure influence survival or recurrence in resected non-small-cell lung cancer? Lung Cancer 1999;25:33-8. [Crossref] [PubMed]
- Sensaki K, Kikuchi K, Kase K, et al. Result of surgical treatment on interlobar pleural invading (p3) lung cancer. Jpn J Thorac Surg 1990;4:765-8.


