Chronic kidney disease and the severity of coronary artery disease and retinal microvasculature changes: a cross-sectional study
Introduction
Chronic kidney disease (CKD) is a major public health problem worldwide, currently estimated to affect more than 10% of the adult population globally (1). It is an independent risk factor for the development of coronary artery disease (CAD), which is the leading cause of morbidity and mortality in the CKD population. Underlying causes may include the combination and interaction of multiple mechanisms including inflammation, oxidative stress, endothelial dysfunction, coronary artery calcification and accelerated atherosclerosis. In an analysis of the Atherosclerosis Risk in Communities Study (ARIC), the incidence of CAD significantly increased with severity of CKD and poor glomerular filtration function (2).
Population-based studies have also shown that retinal microvascular abnormalities, such as retinal arteriolar narrowing and venular widening, are associated with diabetes and hypertension, the two major risk factors for CKD (3-5). Our group has previously reported associations between retinopathy, regular widening and CKD (6). A few prospective studies have also examined the independent association between retinal vessel diameters and CKD, with mixed results (7-9). Assessment of the retinal microvasculature may allow early microcirculatory changes associated with the development of CKD to be examined.
Few published studies have investigated the potential relationship between CAD severity scores versus CKD status. In this present study, we aimed to assess the association between CKD and the severity and extent of CAD; and whether retinal microvascular changes are associated with the presence of renal dysfunction, using data from the Australian Heart Eye Study (AHES) cohort. Between June 2009 and January 2012, data were collected from 1,680 participants who presented to Westmead Hospital, a large tertiary hospital in Sydney, Australia (10-12). Examinations and measurements included a detailed medical history questionnaire, visual acuity testing, biochemical, peripheral blood pressure measurements, invasively measured blood pressure measurements, blood count analysis and retinal photography data. Renal function was assessed according to published National Kidney Foundation guidelines by using the abbreviated Modification of Diet in Renal Disease (MDRD) equation for estimated glomerular filtration rate (eGFR). CKD was defined as eGFR <60 mL/min/1.73 m2 (13).
Methods
Assessment of CAD has been described previously (10,14). Coronary angiograms were scored according to Gensini (severity) and Extent scores. For an assessment of retinal vessel calibre, dilated, digital photographs were taken of the optic disc and macula of both eyes using a Canon 60° fundus camera (Model CF-60DSi, Canon Inc., Tokyo, Japan). Retinal vessel calibres were measured using validated semi-automated software. Ethics approval was obtained from Western Sydney Local Health Network Human Research Ethics Committee. Potential confounders were adjusted for using multivariate analysis. SAS statistical software (version 9.2; SAS Institute, Cary, NC, USA) was used for analyses including t-tests, χ2 tests, linear regression, and logistic regression.
Results
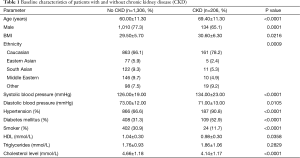
The baseline characteristics of patients without CKD versus those with CKD are summarized in Table 1. Those with CKD were older, more likely to be male and to have a higher body mass index (BMI). They were also more likely to be Caucasian, to have hypertension and diabetes mellitus, and higher high-density lipoprotein and cholesterol levels. However, those with CKD were less likely to be smokers.
Full table
There were no significant associations between CKD status and the extent and severity of CAD, both unadjusted and when adjusted for confounding factors (age, sex, BMI, ethnicity, hypertension, diabetes mellitus and hypercholesterolemia) (Table 2). As well, no significant associations were detected between retinal vessel caliber and prevalent CKD. In unadjusted analyses, a significant association between CKD and narrower retinal arteriolar diameter was observed (P=0.0072). After multivariate adjustment, the association between CKD and retinal arteriolar diameter was attenuated and was no longer significant (P=0.1466). No associations were observed between retinal venular calibre and prevalent CKD (Table 2).

Full table
Conclusions
Thus, in this population-based study, CKD was not associated with either the severity or extent of CAD, nor with retinal arteriolar or venular caliber, when adjusted for relevant confounding factors. Previous studies have documented an association between decreasing renal function and coronary artery calcification. However, whether this association remains significant after adjusting for traditional cardiovascular risk factors is unclear (15-17). The study by Joosen et al. of 2,038 patients, however, showed that after adjustment for traditional cardiovascular risk factors such as smoking, BMI and diabetes mellitus, mild to moderate CKD was not independently associated with coronary plaque burden (16). Similarly, the study by Cho et al. of 4,297 asymptomatic subjects found that after adjustment for proteinuria and other traditional risk factors, there was no significant association between a decrease in eGFR and the risk of obstructive CAD or a high coronary calcium score (>100) (17). These latter findings are in line with our results which did not show an independent relationship between CKD and CAD.
Regarding the association between retinal vessel caliber and CKD, results from prospective studies have been mixed. The ARIC study reported weak associations between arteriovenous (AV) nicking, retinal hemorrhages, microaneurysms, cotton-wool spots, and the likelihood of renal dysfunction development (2,8). The Cardiovascular Health Study (CHS) found strong associations between retinopathy changes and renal function decline, but there were no associations with retinal arteriolar abnormalities such as the arteriolar-venular diameter ratio, AV nicking, or focal arteriolar narrowing (4). We previously reported from the Blue Mountains Eye Study an association of wider venular calibre with CKD (6). There was a suggestion of wider venues in those with CKD in this study but the association was not significant.
Several study limitations need to be considered while interpreting our results. First, the cross-sectional nature of the study limits making causal inferences. Because retinal arteriolar diameters are closely related to hypertension, statistical adjustment for hypertension in the multivariable model may be viewed as an ‘‘overadjustment’’ strategy. Moreover, the limited number of patients with CKD in the present cohort may not have provided adequate statistical power to demonstrate any significant trends or associations. Baseline characteristics and comorbidities were self-reported using medical questionnaires and hence, will involve a certain proportion of recall misclassification.
In conclusion, the present study demonstrated no independent associations between CKD and CAD or retinal vessel caliber in the AHES study. These results warrant validation by future large, prospective longitudinal studies.
Acknowledgements
Funding: The study has been supported by National Health and Medical Research Council (NHMRC) Project (Grant 571012).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Western Sydney Local Health Network Human Research Ethics Committee (Westmead) No. HREC2008/5/4.1 6(2796) AU RED 08/WM EAD/I 22 and written informed consent was obtained from all patients.
References
- Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 2013;382:158-69. [Crossref] [PubMed]
- Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol 2005;16:529-38. [Crossref] [PubMed]
- Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA 2002;287:2528-33. [Crossref] [PubMed]
- Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the Cardiovascular Health Study. Br J Ophthalmol 2002;86:1007-13. [Crossref] [PubMed]
- Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 1999;150:263-70. [Crossref] [PubMed]
- Liew G, Mitchell P, Wong TY, et al. Retinal microvascular signs are associated with chronic kidney disease in persons with and without diabetes. Kidney Blood Press Res 2012;35:589-94. [Crossref] [PubMed]
- Edwards MS, Wilson DB, Craven TE, et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am J Kidney Dis 2005;46:214-24. [Crossref] [PubMed]
- Wong TY, Coresh J, Klein R, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol 2004;15:2469-76. [Crossref] [PubMed]
- Sabanayagam C, Shankar A, Klein BE, et al. Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis 2011;57:682-91. [Crossref] [PubMed]
- Gopinath B, Chiha J, Plant AJ, et al. Associations between retinal microvascular structure and the severity and extent of coronary artery disease. Atherosclerosis 2014;236:25-30. [Crossref] [PubMed]
- Phan K, Mitchell P, Liew G, et al. Relationship between macular and retinal diseases with prevalent atrial fibrillation - an analysis of the Australian Heart Eye Study. Int J Cardiol 2015;178:96-8. [Crossref] [PubMed]
- Phan K, Mitchell P, Liew G, et al. Relationship between macular degeneration with prevalent heart failure: a cross-sectional population study. Int J Cardiol 2015;182:213-5. [Crossref] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1-266. [PubMed]
- Norgaz T, Hobikoglu G, Aksu H, et al. Retinopathy is related to the angiographically detected severity and extent of coronary artery disease in patients with type 2 diabetes mellitus. Int Heart J 2005;46:639-46. [Crossref] [PubMed]
- Cao XF, Yan LQ, Han LX, et al. Association of mild to moderate kidney dysfunction with coronary artery calcification in patients with suspected coronary artery disease. Cardiology 2011;120:211-6. [Crossref] [PubMed]
- Joosen IA, Schiphof F, Versteylen MO, et al. Relation between mild to moderate chronic kidney disease and coronary artery disease determined with coronary CT angiography. PLoS One 2012;7:e47267. [Crossref] [PubMed]
- Cho I, Min HS, Chun EJ, et al. Coronary atherosclerosis detected by coronary CT angiography in asymptomatic subjects with early chronic kidney disease. Atherosclerosis 2010;208:406-11. [Crossref] [PubMed]


