Radiological contribution to the diagnosis of early postoperative complications after lung resection for primary tumor: a revisional study
Introduction
The pulmonary resections performed in patients with primary cancer can be divided into “anatomical” and “non-anatomical” resections. The first one include pneumonectomy, upper bilobectomy (removal of the upper and middle lobes), lower bilobectomy (removal of the lower and middle lobes), lobectomy and segmentectomy or segmental resection (removal of one or more segments). Non anatomical or atypical resections or wedge resections consist of the removal of a limited portion of the lung parenchyma, without following the normal segmental anatomy. In the post-operative course, complications can arise from various types and severity, some potentially lethal, that recognize different pathogenetic mechanisms and differ according to the type of lung resection performed and the time interval between surgical procedure and the onset of complications. Thus, it is accepted to define the “immediate” complications that occur in the first 30 days after surgery, “early” those which occur within the first 6 months, “late” the remaining (1). A further classification, based on the severity, divides complications into “minor” and “major”. The former (non-lethal) include supraventricular arrhythmias, persistent air leak more than 5 post-operative days, accumulation of bronchial secretions with atelectasis and vocal cord paralysis. Major complications (potentially lethal) include post-operative bleeding, pleural empyema with or without bronchial fistula, pneumonia, respiratory failure requiring mechanical ventilation, acute respiratory distress syndrome (ARDS), edema, and pulmonary embolism, ventricular arrhythmias, acute myocardial infarction (AMI) and stroke (1,2). Radiologic studies, X-ray radiography and computed tomography (CT), play an important role in the surveillance of patients undergoing lung resection for primary tumor (3,4). This work aims to describe the incidence, clinical and radiological aspects of immediate complications that may arise after resection for primitive lung neoplasm, with specific reference to those in which imaging is able to provide an effective diagnostic contribution.
Description of immediate complications
In patients undergoing surgical treatment for non-small cell lung cancer (NSCLC) the first post-operative period can be divided into two phases, immediate and early. The two phases differ substantially from each other in relation to the different clinical conditions of the patients and to the different management. In fact, the different clinical conditions and management of patients affects the choice of the radiological technique. In the immediate post-operative course, study of the patient is carried out essentially in the ward and it is therefore limited—both in normal course or in the presence of complications—in obtaining radiographs, performed with portable equipment (only in selected cases can be employed chest sonography or CT); the radiologist plays a central role in the early diagnosis of post-operative complications (5). Patients who underwent greater anatomic resection (pneumonectomy, bilobectomy and lobectomy) are monitored radiologically initially with bedside chest radiographs, and then with the two standard chest radiographs acquired with the patient in the upright position. The timing provides a radiological control in first and second post-operative day, then at the removal of the former and second drainage (after lobar or bilobar resection) in fourth and fifth post-operative day, at discharge and then at varying intervals depending on the clinical evolution. In patients undergoing sublobar resection (segmental or atypical) monitoring is less frequent, with X-rays performed in first post-operative day, at removal of the single drainage (3–4 post-operative days), at discharge and later in accordance with the clinical evolution. Not infrequently, the radiographic finding is difficult to be interpreted and the distinction between normal evolution and the onset of post-surgical complications may be uncertain. Thus, CT is performed in the presence of clinical and/or confounding radiographic conditions or in the absence of a response to established treatment (6). In any case, for a proper interpretation of findings it is essential that the radiologist is provided with all the clinically relevant informations and that the radiologist maintain with the surgeon a continuous cooperation.
Will be discussing cardiorespiratory complications of radiological interest that are seen more often in the immediate post-operative period in patients undergoing pulmonary resection for lung cancer, analyzing both the clinical and radiological issues (Table 1).
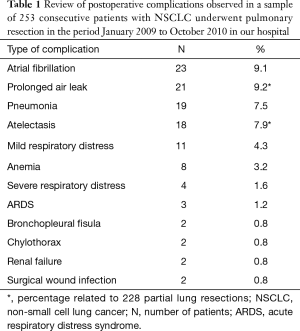
Full table
Persistent air leak
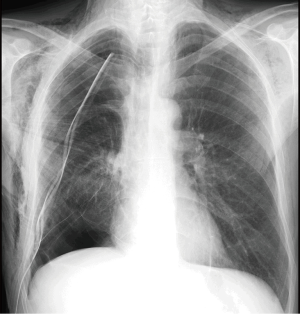
In the absence of a dehiscence of bronchial suture, the air leak observed after partial pulmonary resection results from small parenchymal gaps produced during surgery, typically in the lysis of pleuroparenchimal adhesions or in the interlobar fissures completion. An air leakage is observed in 25% of cases in first post-operative day and in 20% of cases in second post-operative day (7). The air leakage is defined as persistent or prolonged lasting beyond 5 days and shows an incidence ranging from 5% to 10% (7). The main factors which contribute to a persistent air leak are the presence of incomplete interlobar fissures or absent and pulmonary emphysema (1,4), while interventions at greater risk of persistent air leakage are lobectomy of the upper lobe, in particular the right upper lobectomy, for which the reported incidence in the literature varies 49–66% (8) and the bilobectomy (9). The air leakage is evident by observing the pleural drainage; it may be associated with subcutaneous emphysema and rhinolalia (10). As there is no direct radiographic signs of prolonged air leak, the chest X-ray and CT findings may show a substantially regular iconography or highlight an incomplete re-expansion of the residual lung parenchyma, especially in cases of gross air leakage, and a mediastinal and/or subcutaneous emphysema of varying amounts (11) (Figure 1).
Atelectasis
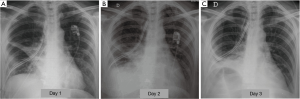
Atelectasis is a common post-operative complication, occurring in 5–10% of cases (11), due to shallow breathing and impairment of the cough mechanism, which afflict patients underwent thoracotomy and promote alveolar hypoventilation and retained secretions within the bronchial lumen. The reduced or absent ventilation of the lung parenchyma causes the progressive resorption of the alveolar gas and the appearance of a right to left shunt, which causes arterial hypoxemia. The distension of the adjacent areas normally ventilated may partially compensate for the loss of volume resulting in atelectasis. However, in case of extended collapse, the diaphragm is elevated, the chest wall is retracted and the mediastinum is displaced towards the affected side. The post-operative atelectasis is seen more often after pulmonary resections involving the execution of the bronchial anastomosis (i.e., sleeve lobectomy) (11), particularly after resection of the lower lobes, since the anastomosis between the remaining upper lobar bronchus and the main bronchus may present an abnormal angle (kinking of the anastomosis) (12). Clinical examination detects noises from stagnation of secretions, which can gradually evolve into respiratory silence, bronchial murmur and percussoria obtuseness. The stagnation of secretions can promote superinfection of atelectasis and pneumonia. Radiographically signs that show an abnormal loss of volume of operated hemithorax (dislocation of mediastinal structures toward the same side and/or raising of corresponding hemidiaphragm), associated with a lobar or segmental cuneiform opacity, without air bronchogram, are highly suggestive (3) (Figure 2).
Pneumonia
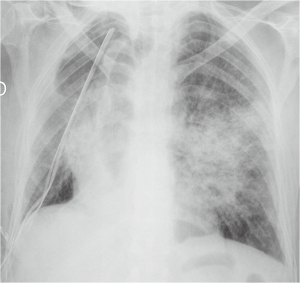
The post-operative pulmonary infection is observed in a proportion of 2–22% of patients undergoing partial or total lung resection (4). Among the risk factors there are a prolonged preoperative hospitalization, immunosuppression, type of resection, reduced functional breathing reserve, cigarette smoke and, as already mentioned, the post-operative atelectasis (11). Inhalation and bacterial colonization of the atelectasic parenchyma are the most common immediate causes (5). The classic diagnostic criteria include hyperthermia >38 °C, the emission of purulent sputum, isolation of pathogenic organisms in sputum or in bronchial aspirates and the appearance of one or more inflammatory opacities at radiographic examination of the chest. The onset of clinical manifestations may sometimes precedes the appearance of evident radiographically alterations (5). At radiographic examination pneumonia presents as a parenchymal opacity, in typical cases with air bronchogram, generally with lobular pattern, without a typical lobar or segmental distribution (Figure 3). The appearance of excavation is a sign suggestive of necrotizing evolution of the disease (3).
ARDS
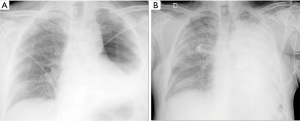
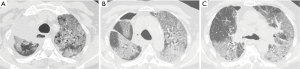
With the acronym ARDS it is shown an acute respiratory failure, mostly between the first and fifth post-operative day, characterized by tachycardia, tachypnea, and hypoxemia refractory to administration of O2 [see new validated Berlin definition (13)]; however ARDS may also occur in association with other complications such as pneumonia or bronchopleural fistula (14). Respiratory failure that follows requires for patient intensive care and mechanical ventilation. The prognosis remains serious, although the frequency and mortality of ARDS are difficult to quantify because of the variety of nomenclatures given to this condition (“pulmonary edema after pneumonectomy with no identifiable cause”; “edema formation following pneumonectomy characterized by normal cardiac filling pressures, high pulmonary artery pressures and high cardiac output”; “severe and often lethal respiratory failure secondary to noncardiac high permeability pulmonary edema after resection of the lung”; “noncardiogenic edema complicating lung resection”) (3-5,15). The variety of denominations certainly reflects the multiplicity and complexity of the risk factors and pathophysiological mechanisms. The overall incidence of ARDS, observed after pulmonary resection varies from 2% to 15% (15). Lung injury may occur as a result of multiple insults, direct or indirect. The possible causes of ARDS include: excessive fluid infusion during intra- and immediate post- operative course, the interruption of lymphatic network (especially after pneumonectomy), the variation of pressure in the post-operative time in pulmonary capillary, direct damage to capillary endothelium due to intra-operative positive pressure mechanical ventilation (volutrauma for single lung ventilation), post-operative dysfunction of the right ventricle. Recent studies seem to suggest that the increase in endothelial permeability plays a central role in the pathogenesis of this condition. The exact mechanism of endothelial damage is yet to be defined, but it is believed that the stress produced by ischemia-reperfusion injury secondary to the temporary suspension of ventilation in the lung is mediated by reactive oxygen species (ROS), group of oxidant metabolites, radical and non-radical species, highly reactive (15). The chest radiograph is more often negative in the first 24 h after the onset of clinical manifestations of respiratory instability. Seriated exams show, however, the rapid development of extensive opacities homogeneous or with asymmetric and “patchy” distribution, without sparing the periphery of the mid or upper lungs like hydrostatic pulmonary edema (16,17) (Figure 4). A CT scan reveals areas of consolidation such as ground-glass opacities, with greater visibility of the interlobular septa, associated with areas of parenchymal consolidation, mainly located in the declivous lung zones (gravitational distribution of lesions) (16) (Figure 5).
Pulmonary edema
The most common cause of lung edema observed after pulmonary resection is the excessive fluid infusion during surgery and in the immediate post-operative period. Contributing factors include the baro/volutrauma related to anesthetic procedures of exclusion and re-ventilation of lung and surgical manipulation of the lung itself (1). It is characterized by the rapid onset of dyspnea and hypoxemia, secondary to pulmonary interstitium imbibition, which can evolve in respiratory failure. Clinically wet sounds are detected, with little or no change with cough. Radiological more frequently aspects in mild forms of pulmonary edema following partial or total parenchymal resection are similar to those of hydrostatic edema without “diffuse alveolar damage” and include Kerley lines of type B, peribronchial cuffing and ill-defined vessels; these features have a tendency to disappear within a few days. In the most severe cases, edema is manifested by the appearance of diffuse infiltrates, which is identical to that observed in the course of ARDS (4,18).
Bronchopleural fistula
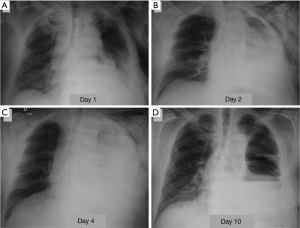
The dehiscence of the bronchial suture can manifest in the first post-operative course so as years after surgery, more often after pneumonectomy (with a frequency of 9% after right pneumonectomy and of 2% after left pneumonectomy). The incidence is very low after lobectomy (about 1%) or segmentectomy (approximately 0.3%) (1,19). Among the risk factors to be considered, in addition to the type of resection, pre-operative chemotherapy and/or radiotherapy, excessive devascularization of the bronchial wall secondary to lymphadenectomy, the infection of the suture line, immunosuppression, diabetes. An early bronchopleural fistula should be suspected in the presence of a major air leakage from the drainage, new onset or increased sharply compared to previous days. More often the patient complains of productive cough of mucopurulent sputum, sometimes streaked with blood, and dyspnea, which may be associated with manifestations of hemodynamic instability and shock. Subcutaneous emphysema may be present (11). The radiologic findings that raise suspicion of bronchopleural fistula are characterised by a progressive increase of a residual pleural space (at the expense of a reduction of the liquid component), by the appearance of an air-fluid level in a hemithorax previously entirely radiopaque (Figure 6), the development of a tension pneumothorax, a reduction of a pre-existing air-fluid level more than 2 cm during the first post-operative course (5). CT may allow direct demonstration of pathological communication of airways with pleural space (3).
Empyema
It is observed more often after pneumonectomy, with an estimated incidence of 2–16%; the main risk factor is the bronchopleural fistula (20). It is less common after lobectomy and in this case it is often associated with the presence of residual pleural space. It is characterized by fever, deterioration of general condition, respiratory failure, elevated inflammatory markers, septic state. The period of onset can vary from a few days to months or even years after surgery; thoracentesis is fundamental for the definitive diagnosis (detection of serum-corpusculated liquid or frankly purulent). After pneumonectomy, radiographic signs of empyema are represented by the rapid clouding of the residual cavity or, conversely, by the over-distension of a post-surgical space previously radiopaque, with deviation of the mediastinum. Even the appearance of an air-fluid level in a residual already opacified space may be indicative of empyema, supported by gas producers microorganisms or, more often, by a bronchopleural fistula (5). After lobectomy, chest radiographs show the appearance or increase in size of a pleural space with air-fluid level. CT is the examination of choice for the suspicion of post-pneumonectomy empyema; CT signs are represented by a convex appearance of the mediastinal margin of the residual cavity (which is usually concave) in the absence of deviation of the mediastinum, a thickening and enhancement of the parietal pleura and the presence of air-fluid levels within the residual cavity (3,21).
Chylothorax
Chylothorax is defined as the accumulation of chyle in the pleural cavity, due to injury of the thoracic duct. The reported incidence in the literature varies from 0.04% to 2% after lobectomy (22,23) and from 0.7% to 1% after pneumonectomy (5). The systematic lymphadenectomy is the main risk factor, especially in patients undergoing pre-operative chemotherapy (1). The anatomical areas at greatest risk of injury of the lymphatic system are: the paravertebral lower region of right hemithorax in extrapleural resection, the subaortic region, the subcarinal region and pulmonary ligament area during systematic lymphadenectomy (22). The radiographic manifestations of postoperative chylothorax, completely non-specific, are related to the appearance or the rapid increase of pleural effusion. It contains protein as well as fat and CT shows almost the same density of water, rarely it has an attenuation lower than 0 HU (hounsfield unit).
Hemothorax
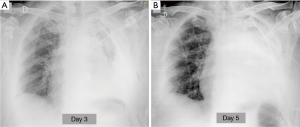
In the absence of deficiency of coagulation, the post-operative bleeding has an extremely low incidence, lower than 1% (1). It is often the result of inadequate hemostasis of bronchial artery or of vessels of the chest wall, more rarely the failure of the ligature or suture of a major pulmonary vessel, which would be rapidly lethal. A blood loss greater than 200 mL/h identifies a major bleeding, which requires surgery approach (11). In addition to blood loss from drainages clinical signs of acute hypovolemia are detectable (low blood pressure, tachycardia, cold or clammy skin, contraction of diuresis). Radiographically hemothorax occurs, in a non-specific manner, with the onset and rapid growth of a pleural effusion (3) (Figure 7). CT reveals a high density liquid (>50 HU), often heterogeneous for the presence of clots and septa or characterized by fluid-blood level, with more dense component deposited (4).
Other complications, that can occasionally be observed after pulmonary resection for primary tumour, include the following.
Cardiac herniation
It is a rare complication that occurs after intrapericardial pneumonectomy (with ligation or suture of the pulmonary vessels within the pericardial sac), most often to the right, and it is due to herniation of the heart through a pericardial gap (5,21). The symptomatology is related to the sudden interruption of venous return to the heart secondary to the angle of the vena cava. To the right, when the heart rotates counterclockwise on the axis, acute superior vena cava syndrome appears with massive edema of the upper regions of the body and an acute heart failure. The radiographic signs include, in cardiac herniation toward the right side, displacement of the cardiac apex in the lateral costophrenic sulcus (if the heart rotates clockwise) or in the posterior costophrenic sulcus (if the heart rotates counterclockwise). In herniation toward the left you can appreciate a hemisphere-shaped left border with an incisura between the great vessels and the more laterally herniated cardiac margin (24).
Lung torsion
It consists of the rotation of part or all of the residual lung parenchyma on the hilar structures (5). It occurs most frequently in the middle lobe after right upper lobectomy. It is facilitated by the presence of a large complete fissure and determines hemorrhagic infarction and atelectasis of lobar parenchyma. If it is not identified and treated promptly, consequent superinfection requires removal of the middle lobe (1). On radiograph it may be suspected in the presence of a pulmonary condensation with rapid onset and with lobar distribution, associated with an increase of volume of the involved lobe (3). The CT signs of the lobar torsion are represented by a rapid and progressive volumetric increase of involved lobe which presents a reduced enhancement and shows in its context areas of consolidation of the ground-glass type, accompanied by interstitial thickening; at hilar level it is documented obstruction of the arterial branch or branches and of lobar corresponding bronchus (4). The torsion of the entire lung is a even more rare complication, with a very high mortality if not promptly recognized and treated. It determines reflux in the pulmonary veins, causing pulmonary edema and inadequate venous return to the heart with shock by low flow (4).
Bronchovascular fistula
It is a rare event, usually due to asymptomatic infection of the bronchial suture line which erodes the adjacent pulmonary artery; to the close proximity of the anatomical structures involved it is more frequent after left upper lobectomy (4). After sleeve lobectomy dehiscence of bronchial anastomoses can lead to the formation of an abscess that involves the nearby arterial wall. The erosion of this is manifested by hemorrhage, often fatal.
Esophagopleural fistula
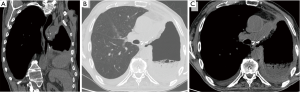
It is a rare complication of pneumonectomy. It occurs mostly on the right, in the section immediately below the carina, and it is favored by devascularization of the esophageal wall secondary to surgical procedures (for instance, an extended lymphadenectomy) (1). If it is caused by the direct iatrogenic injury of the esophageal wall, clinical manifestations appear in the immediate post-operative course: diagnosis is clear when food is discharged through the pleural drainage. Minor esophagopleural fistulas can be difficult to diagnose, and tend to cause a chronic empyema (4). Late fistulas may instead be caused by a tumor recurrence or chronic inflammation in the esophagus, the bronchial stump or in the surrounding tissues (22). Conventional radiography has clear limitations: esophagopleural fistula in fact can not be diagnosed until air appears into the pleural cavity. The esophagography continues to play an important role in the diagnosis of esophagopleural fistula. Water-soluble contrast medium must be administered orally (not via catheters). In the upright position, however, it is not always achieved the opacification of the fistula, particularly if it is small. It is therefore recommended that the patient is examined also in the lateral decubitus position on the operated side (25). CT, especially if it is performed in very thin layers, adds more detailed informations, it helps the therapeutic management and allows (in case of late onset) to recognize a recurrence of the tumor that may be responsible for the fistula (4) (Figure 8).
Conclusions
The radiologist plays a major role in the diagnosis of the complications that occur after pulmonary resection for primary tumor in the immediate post-operative course. The relatively low diagnostic accuracy of the chest X-ray needs that the radiologist acquires a thorough knowledge and familiarity with the various types of intervention and related clinical problems and, above all, with the radiographic findings to be considered “normal” and with the different radiological patterns that occur in complications. To this aim, it is useful a close collaboration with the surgeon. The constant comparison with previous chest X-rays is also of great importance for the correct interpretation of radiographic findings as some pathological manifestations may reveal only by time assessment, such as in the diagnosis of a bronchopleural fistula. When the radiological findings prove misunderstandings or a clear clinical radiological discrepancy emerges, CT is able to provide additional information, often more timely and accurate than the conventional radiology.
Acknowledgements
We would like to thank Prof. Cesare Fava for the suggestions received in the choice of topic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alloubi I, Jougon J, Delcambre F, et al. Early complications after pneumonectomy: retrospective study of 168 patients. Interact Cardiovasc Thorac Surg 2010;11:162-5. [Crossref] [PubMed]
- Pei G, Zhou S, Han Y, et al. Risk factors for postoperative complications after lung resection for non-small cell lung cancer in elderly patients at a single institution in China. J Thorac Dis 2014;6:1230-8. [PubMed]
- Feragalli B, Mantini C, Mereu M, et al. Come interpretare l’imaging toracico post chirurgia toracica e cardiaca. Radiol Med 2010;115:S45-61.
- Kim EA, Lee KS, Shim YM, et al. Radiographic and CT findings in complications following pulmonary resection. Radiographics 2002;22:67-86. [Crossref] [PubMed]
- Pool KL, Munden RF, Vaporciyan A, et al. Radiographic imaging features of thoracic complications after pneumonectomy in oncologic patients. Eur J Radiol 2012;81:165-72. [Crossref] [PubMed]
- Perez JA, Nistal MS, Charterina SA, et al. Role of MDCT in the evaluation of complications of lung surgery. ECR 2010;10.1594, C-0799.
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Palma A, Lopez G, Loenches N, et al. Postoperative evolution of lung cancer and its complications: what we should know. ECR 2010;10.1594, C-0766.
- Cerfolio RJ. Early postoperative complications. In: Patterson GA. editor. Pearson's Thoracic and Esophageal Surgery, Third Edition. Philadelphia: Churchill Livingstone, an imprint of Elsevier Inc., 2008:160-5.
- Massard G, Wihlm JM. Postoperative atelectasis. Chest Surg Clin N Am 1998;8:503-28. [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Padley SP, Jordan SJ, Goldstraw P, et al. Asymmetric ARDS following pulmonary resection: CT findings initial observations. Radiology 2002;223:468-73. [Crossref] [PubMed]
- Kutlu CA, Williams EA, Evans TW, et al. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000;69:376-80. [Crossref] [PubMed]
- Webb WR, Higgins CB. Pulmonary edema, the Acute Respiratory Distress Syndrome, and Radiology in the Intensive Care Unit. 2nd edn. Thoracic Imaging: Pulmonary and Cardiovascular Radiology, Philadelphia: Lippincott Williams and Wilkins, 2010:337-9.
- Cardinale L, Volpicelli G, Lamorte A, et al. Revisiting signs, strengths and weaknesses of Standard Chest Radiography in patients of Acute Dyspnea in the Emergency Department. J Thorac Dis 2012;4:398-407. [PubMed]
- Waller DA, Gebitekin C, Saunders NR, et al. Noncardiogenic pulmonary edema complicating lung resection. Ann Thorac Surg 1993;55:140-3. [Crossref] [PubMed]
- Hollaus PH, Setinek U, Lax F, et al. Risk factors for bronchopleural fistula after pneumonectomy: stump size does matter. Thorac Cardiovasc Surg 2003;51:162-6. [Crossref] [PubMed]
- Ng CS, Wan S, Lee TW, et al. Post-pneumonectomy empyema: current management strategies. ANZ J Surg 2005;75:597-602. [Crossref] [PubMed]
- Chae EJ, Seo JB, Kim SY, et al. Radiographic and CT findings of thoracic complications after pneumonectomy. Radiographics 2006;26:1449-68. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Nishimura M, et al. Treatment strategy for chylothorax after pulmonary resection and lymph node dissection for lung cancer. J Thorac Cardiovasc Surg 2002;124:499-502. [Crossref] [PubMed]
- Kutlu CA, Sayar A, Olgac G, et al. Chylothorax: a complication following lung resection in patients with NSCLC—chylothorax following lung resection. Thorac Cardiovasc Surg 2003;51:342-5. [Crossref] [PubMed]
- Tschersich HU, Skorapa V, Fleming WH. Acute cardiac herniation following pneumonectomy. Radiology 1976;120:546. [Crossref] [PubMed]
- Massard G, Ducrocq X, Hentz JG, et al. Esophagopleural fistula: an early and long-term complication after pneumonectomy. Ann Thorac Surg 1994;58:1437-40; discussion 1441. [Crossref] [PubMed]


