Complete response of 7 years’ duration after chemoradiotherapy followed by gefitinib in a patient with intramedullary spinal cord metastasis from lung adenocarcinoma
Introduction
Intramedullary spinal cord metastasis (ISCM) is a rare but serious cancer complication which causes rapid progression of neurological deficits within a period of days to weeks (1-3). Prognosis is poor, with median survival of 4 months (4). Optimum treatment remains controversial. Here we report a patient with ISCM from lung adenocarcinoma, in whom chemoradiotherapy followed by gefitinib was effective for 7 years.
Case report
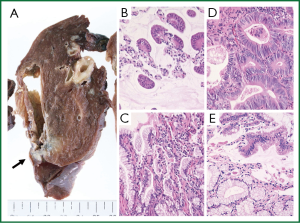
A 35-year-old man underwent a right lower lobectomy in March 2005. He had a smoking history of 15 pack-years. Resected specimens revealed adenocarcinoma, mixed subtypes, bronchioloalveolar > acinar, measuring 2.5-cm in size with 2 nodules of intrapulmonary metastases in the same lobe (Figure 1). The pathological stage was IIB, pT3N0M0 (5). Serum carcinoembryonic antigen (CEA) levels were slightly increased to 9.4 ng/dL, then normalized following surgery. Two cycles of postoperative adjuvant chemotherapy with docetaxel and carboplatin were implemented.
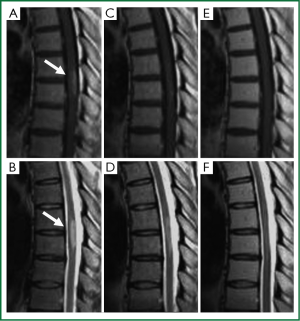
In November, 2005, the patient reported numbness extending from the right hip to the toes upon awakening, which developed into pain in both legs the next day. On admission, sensory disturbance of Th10 or lower was observed. Magnetic resonance imaging (MRI) revealed an intramedullary spinal cord tumor at Th7/8, showing a high-intensity area with fusiform bulging in T2-weighted images and a gadolinium-enhanced core in T1-weighted images (Figure 2A,B). Positron emission tomography, serum CEA level and cerebrospinal fluid cytology were all negative. Surgical resection was offered but rejected. The symptoms progressed further and urinary incontinence began two weeks later. Gait disturbance occurred due to reduced tendon reflexes.
In December 2005, treatment was initiated with high dose steroids, followed by concurrent chemoradiotherapy consisting of irradiation (35 Gy in 13 fractions), vinorelbin and cisplatin. Although radiotherapy resulted in transient improvement, the symptoms worsened again during the 2nd cycle of chemotherapy (Figure 2C,D). In February 2006, gefitinib was administered. Symptoms improved after 2 weeks. The patient was discharged one month later, able to walk with slight numbness in both legs. Two months later, gadolinium-MRI showed no areas with abnormal contrast (Figure 2E,F). EGFR genetic analysis of the primary site was carried out retrospectively, but no activating mutations were found. The patient has remained in complete response for 7 years on gefitinib.
Discussion
MRI is sufficiently sensitive to detect intramedullary lesions, but the findings are not specific in that T2-weighted images are of high intensity for tumor and surrounding edema, and gadolinium-enhancement delineates central tumor. However, ISCM cannot be distinguished from primary spinal cord neoplasms or other non-neoplastic lesions such as demyelinating plaques, radiation myelitis, or paraneoplastic myelopathy on the basis of these MRI findings (4,6,7). The clinical course with rapid deterioration of neurological symptoms distinguishes ICSM from primary intramedullary tumors, which typically present with a slower progression of symptoms (2-4,6). In three-quarters of reported patients, the time from first onset of neurological symptoms to development of full neurological deficits is <1 month (2,3). Pain and weakness are more common early in the course, with sensory loss reported after. Bowel and bladder dysfunction most typically present later (6).
ISCMs constitute 1% to 3% of all intramedullary tumors. Lung cancer is the most frequent primary tumor (48%), followed by breast cancer (16%). At presentation, 26% of patients with ISCM have no identified primary tumor; most of these patients undergo surgery for decompression and diagnosis. In the remaining three-quarters, the primary is known. Most surgeons are reluctant to undertake aggressive resection because of the inherent risks of spinal cord operations and the advanced state of the systemic malignancy (4). Combination radiotherapy, steroids and chemotherapy is frequently applied, but efficacy depends on the sensitivity of the primary tumor. Surgical treatment might be considered for patients with radioresistant single metastases in the early stage of disease not yet with serious neurological deficits, and in the absence of multiple systemic metastases (4,8). However, survival benefit seems marginal. Median survival was 6 months in surgically-treated patients, 5 months in conservatively-treated patients, and one month for palliation. Functional improvement was observed in 58% of surgically treated patients, whereas 11% deteriorated. In patients treated with a conservative regimen, 21% improved, 63% showed no change, and 17% deteriorated (4). ISCM has been frequently reported as well-circumscribed and amenable to gross total resection (1,9-11). On the other hand, infiltrative (12) or indistinct (6) margins, heavy bleeding (13,14), and disappointing postoperative course (14) has been reported, especially in ISCM from lung cancer. Thus, resectability might be determined by primary lesion histotype.
The present patient received chemoradiotherapy with steroids, followed by gefitinib, and has maintained a complete response(CR) for 7 years. Several uncertainties remain concerning diagnosis and treatment efficacy. The diagnosis of ISCM seems reliable, with the rapidly progressing neurological symptoms, which improved transiently after radiotherapy, then progressed again, but finally dramatically improved two weeks after starting gefitinib. Although later effects of radiation injury or edema cannot be excluded, the clinical course suggested that gefitinib was responsible for the favorable outcome. Despite lack of EGFR mutations in the primary lesion, they might have been present in the metastases (15). Five years after starting gefitinib, we considered withdrawal, but the patient wished continue and has now maintained a CR with excellent physical status for 7 years.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kalayci M, Cağavi F, Gül S, et al. Intramedullary spinal cord metastases: diagnosis and treatment - an illustrated review. Acta Neurochir (Wien) 2004;146:1347-54; discussion 1354.
- Grem JL, Burgess J, Trump DL. Clinical features and natural history of intramedullary spinal cord metastasis. Cancer 1985;56:2305-14.
- Mut M, Schiff D, Shaffrey ME. Metastasis to nervous system: spinal epidural and intramedullary metastases. J Neurooncol 2005;75:43-56.
- Sung WS, Sung MJ, Chan JH, et al. Intramedullary Spinal Cord Metastases: A 20-Year Institutional Experience with a Comprehensive Literature Review. World Neurosurg 2012. [Epub ahead of print].
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71.
- Connolly ES Jr, Winfree CJ, McCormick PC, et al. Intramedullary spinal cord metastasis: report of three cases and review of the literature. Surg Neurol 1996;46:329-37; discussion 337-8.
- Schiff D, O’Neill BP. Intramedullary spinal cord metastases: clinical features and treatment outcome. Neurology 1996;47:906-12.
- Grasso G, Meli F, Patti R, et al. Intramedullary spinal cord tumor presenting as the initial manifestation of metastatic colon cancer: case report and review of the literature. Spinal Cord 2007;45:793-6.
- Findlay JM, Bernstein M, Vanderlinden RG, et al. Microsurgical resection of solitary intramedullary spinal cord metastases. Neurosurgery 1987;21:911-5.
- Kaya RA, Dalkiliç T, Ozer F, et al. Intramedullary spinal cord metastasis: a rare and devastating complication of cancer--two case reports. Neurol Med Chir (Tokyo) 2003;43:612-5.
- Ogino M, Ueda R, Nakatsukasa M, et al. Successful removal of solitary intramedullary spinal cord metastasis from colon cancer. Clin Neurol Neurosurg 2002;104:152-6.
- Sutter B, Arthur A, Laurent J, et al. Treatment options and time course for intramedullary spinal cord metastasis. Report of three cases and review of the literature. Neurosurg Focus 1998;4:e3.
- Raco A, Delfini R, Salvati M, et al. Intramedullary metastasis of unknown origin: a case report. Neurosurg Rev 1992;15:135-8.
- Watanabe M, Nomura T, Toh E, et al. Intramedullary spinal cord metastasis: a clinical and imaging study of seven patients. J Spinal Disord Tech 2006;19:43-7.
- Kalikaki A, Koutsopoulos A, Trypaki M, et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923-9.


