Division of the intersegmental plane during thoracoscopic segmentectomy: is stapling an issue?
Introduction
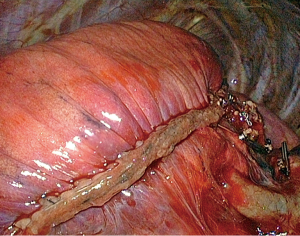
Although pulmonary lobectomy remains the standard treatment of operable early stage non-small cell lung cancer (NSCLC) (1), the interest for anatomic segmentectomy has grown over the past years as many elderly and/or fragile patients cannot tolerate a lobectomy but can undergo a sublobar resection, especially if performed without thoracotomy (2). For patients presenting with metastases or benign lesions, the need for sparing valid parenchyma is even more important and a thoracoscopic limited sublobar resection is more and more considered as the procedure of choice. However, segmentectomies are considered technically challenging, especially when performed by thoracoscopy. One of the most problematic steps of the operation is the division of the intersegmental plane. As the surgeon cannot rely on digital handling as he/she would do during an open peeling technique, stapling the demarcation line is becoming the preferred method. However, the use of stapling is criticized as it can plicate the parenchyma of the preserved segments (Figure 1), leading loosing valid parenchyma and impairing pulmonary re-expansion (3). Asakura et al. noted that stapling interferes with the expansion of preserved lung in comparison to mere cutting with scissors due to the visceral pleura being caught in the staple line (3). It is unclear whether this issue has any impact on the postoperative course, some authors claiming they did not noticed any difference when comparing stapling to other methods of dividing the intersegmental plane (4). For this reason, we have analyzed two postoperative features, i.e., postoperative air leak and pulmonary re-expansion, in our series of thoracoscopic segmentectomies where the intersegmental plane was divided with an endostapler.
Methods
The records of a consecutive series of 175 thoracoscopic anatomic pulmonary segmentectomies were reviewed. The study was approved by our Institutional Review Board N° 2012-10.
Technical aspects
A full thoracoscopic approach was used to perform all segmentectomies of this series, according to a previously reported technique (5). Briefly, we used a deflectable scope housing a distal charged-coupled device connected to a high-definition camera. Only endoscopic instruments were used. These were inserted through 3 to 4 trocars, according to the necessity of dissection or exposure. No utility incision was used. On completion of the pulmonary resection, the specimen was placed into an endobag and retrieved through one of the port sites that was enlarged to a length of 2 to 4 cm, depending on specimen size. The control of large vessels was accomplished with endostaplers while haemostasis of small-calibre vessels was performed with clips or with a bipolar vessel-sealing device or with a combination of both. Demarcation between the resected and preserved segments was made possible by re-ventilation. The demarcation line was marked with cautery dots and a dedicated long and narrow lung forceps was applied on the parenchyma, in order to compress it and ease the application of the stapler. In all patients division of the intersegmental plane and of the fissure was made using endostaplers of two types: before 2011 regular 4.8 mm staples (Endo-GIA, Covidien©, Plymouth, MN, USA) were used while after 2011, cartridges including staples ranging from 4.3 to 5.0 mm (Tri-stapled technology, Covidien©) were used.
For patients operated on for lung cancer, intersegmental lymph nodes, if present, were analysed by frozen section to confirm segmentectomy (if malignant, the procedure was converted into a lobectomy). An additional radical mediastinal lymphadenectomy was performed in all patients. The bronchus stump and portion of the staple line adjacent to the nodule were also examined by frozen section if close to the tumour. If margins were positive or doubtful, the procedure was transformed into lobectomy. Those patients are not included in this study.
Data analysis
The series was analyzed retrospectively from a prospectively collected data which was approved by our IRB (CEPAR 2013-002) including demographic criteria, type of resection, diagnosis, duration of surgery and blood loss, chest tube duration, hospital stay duration and complications. Lung re-expansion assessment included chest roentgenograms at discharge and at one-month consultation. The chest X-rays of each patient were included into the database and analyzed by an independent observer. Digital radiography (Picture-Archiving Communication System, PACS) workstation was used for image review. Cursor auxiliary function enabled to estimate the shift of the pleural line. The size of pneumothorax was determined as the measure of the longest axis from lung margin to chest wall.
Statistical analysis
The statistical analysis was performed using SPSS 17.0 software (Chicago, IL, USA). Unless otherwise specified, continuous variables were expressed as mean + range and categorical variables as percentages. Between-group comparisons were performed using the χ2 test for qualitative variables or Fisher’s exact test when needed. To compare multiple categorical variables one-way analysis of variance was adopted. A P value <0.05 was considered statistically significant.
Results
Between 2007 and 2014, 175 thoracoscopic anatomic segmentectomies were performed in our department. Out of these patients, 10 (5.7%) were excluded from our study because of conversion to thoracotomy for the following reasons: vascular injury in 6 cases, nodule not found in 3 patients, and ischemia of adjacent segments in 1 patient.
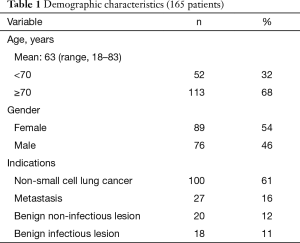
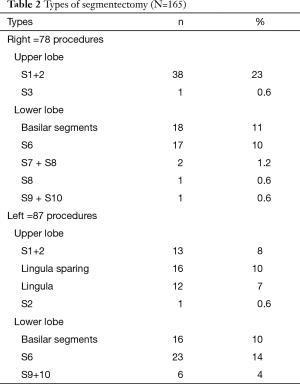
Among the 165 remaining patients, there were 89 females (54%) and 76 males (46%), with a mean age of 63 years (range, 18–83 years). Most of the patients (n=113, 68%) were 70 years or older. Indications for segmentectomy were as follows: primary lung cancer (n=100, 61%), metastases (n=27, 16%), benign lesions (n=20, 12%), and infectious lesions (n=18, 11%) (Table 1). The type of segmentectomy is reported in Table 2. We used the nomenclature of segmentectomies as described by Nomori and Okada (6). Intraoperative adverse events occurred in 8 (5%) patients. There were four cases of stapling line disruption that required endoscopic suturing, all occurring with the use of regular 4.8 mm staples (no disruption was noticed after the introduction of black cartridges with 3-staples technology), 1 case of venous injury during a S7+8 segmentectomy, 1 arterial branch injury caused by the application of a vascular clip. All these adverse events complications were managed thoracoscopically. There were two conversions from a planned S8 and S7+8 resection to a basilar segmentectomy because of invaded resection margin at frozen section. The overall postoperative complication rate was 17%. There was 1 (0.6%) major complication consisting of acute respiratory failure due to pulmonary edema requiring non-invasive mechanical ventilation. Minor complications occurred in 27 patients (16%) and were mainly of respiratory order: 7 prolonged air leaks (PALs), 3 pneumothorax, 3 pneumonia, 2 atelectasis requiring bronchoscopy, 2 subcutaneous emphysema and other complications in 10 (4 atrial fibrillation, 2 renal failure, 2 pulmonary embolism, 1 hepatic failure and 1 thrombocytopenia). The mean duration of surgery was 162 minutes (range, 50–315 minutes) and the mean blood loss was 74 mL (range, 0–450 mL). The mean drainage duration was 3 days (range, 1–13 days) and mean stay 5.7 days (range, 2–22 days).
Full table
Full table
Prolonged air leaks
PALS, defined as air leaking lasting 7 or more days, are observed after any type of lung resection. They can be related with the condition of pulmonary parenchyma or with technical issues. In order avoiding underestimation of our complication rate, we did consider that all PALs could be more or less related to the method used for division of the intersegmental plane. There were 7 PALs (4.2%), with a maximum chest tube duration of 13 days. None of the PALs required pleurodesis or reoperation.
Lung re-expansion
All patients had a chest roentgenogram at discharge. A not fully re-expanded lung was observed and measured in 10 patients (6%) as follows: 7 pneumothorax and 3 apical pleural effusions with measured average size of 2.2 cm (range, 1.1–4.1 cm).
At follow-up at 1 month, 3 patients were controlled at their referral institution and no chest X-ray was available. Out of the remaining 162 patients, there were only 4 cases of apical cap (2.5%), whose average size was 2.3 cm (range, 1.2–3.9 cm) (Table 3). All these 4 patients had prior non re-expanded lung at discharge.

Full table
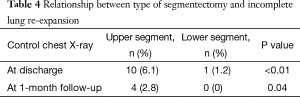
Patients who underwent an upper lobe segmentectomy presented significantly more frequently with an incomplete re-expansion both at discharge (P<0.01) and at 1-month follow-up (P=0.04), compared to patients with lower lobe segmentectomy (Table 4). Descriptive data concerning the type of segmentectomy and measure of incomplete lung re-expansion are reflected in Table 5. In addition, on univariate analysis, the mean drainage duration was significantly longer (P<0.01) in patients who underwent upper segmentectomy (mean 3.7 days; range, 1–13) than those who underwent lower segmentectomy (mean 2.7 days; range, 1–5).
Full table
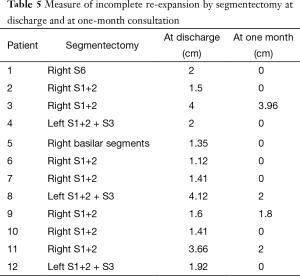
Full table
Discussion
Although lobectomy remains the gold standard resection in most NSCLC, many publications have stressed the interest of sublobar resection in ground glass opacities (GGO) and in clinical stage Ia carcinomas, especially in elderly and/or fragile patients (7). Sublobar resections spare more parenchyma than lobectomies and their postoperative course is usually easier and faster (2,8). In some metastatic lung diseases (9) and benign lesions (10) that are not amenable to wedge resection because they are deeply located or because of their close relationship to bronchovascular elements, an anatomic segmentectomy is the method of choice. Several studies have demonstrated that the outcome of sublobar resections is even better when the procedure is performed thoracoscopically. We have shown that a thoracoscopic approach for segmentectomy resulted in a significantly shorter hospital stay and lower rate of complications (11). Similar results have been recently reported (2) and it has also been demonstrated that the thoracoscopic approach allows operating with a much lower FEV1 than the one usually accepted for a thoracotomy (7).
However, mastering thoracoscopic segmentectomy can be technically challenging and identifying the anatomy and the intersegmental plane is of concern (12,13). Once the demarcation line has been identified, choosing the adequate method of division can also be a dilemma. The conventional way of dividing the intersegmental plane is to cut the parenchyma along the segmental vein. Until now, cutting was performed with electrocautery (and more recently with an electrothermal bipolar vessel sealing device), reinforced or not with fibrin sealant or covered or not with a mesh. This technique is supposed to preserve the parenchyma but is at risk of bleeding and air leaking. The rapid development of the thoracoscopic approach has favored the stapling method, which is considered as more straightforward. Stapling seems safer but could result in some loss of valid parenchyma with impaired lung re-expansion (3).
Studying this issue is difficult as there are few objective facts to compare. The studies can be based on experimental data obtained from ex vivo tissues, or on the measurement of pulmonary function, or on specific postoperative complications (mainly PALs) or on radiologic measurements of lung re-expansion as we did in this study.
Air leakage
Although stapling may have some limitations, it seems it causes less air leaks. In our series, we noticed 7 prolonged air leaks (4.2%) but none of them required pleurodesis or reoperation. All of them had predictive factors of prolonged air leakage (emphysema n=3, tuberculosis n=1, incomplete fissure n=3). Miyasaka et al. compared electrocautery and the use of staplers for dividing the intersegmental plane (4). All segmentectomies were performed by an open approach. Sample size was 49 patients, with 18 patients in the stapling group and 31 in the electrocautery group. Prolonged air leaks were observed in 4 patients (8.2%), all in the latter group (P=0.005). Three of these 4 patients had to undergo a chemical pleurodesis and one of them was reoperared for closing the air leak. In a series of 102 hybrid video assisted thoracic surgery (VATS) segmentectomy, electrocautery alone, i.e., without stapler, was used to divide the intersegmental plane and a fibrin sealant was applied along the raw surface of the remaining lung to prevent air leakage. PALs were observed in 4 patients (3.9%) and 3 (2.9%) had a late alveolo-pleural fistula requiring tube drainage (14). In our series, none of the 7 PALs (4.2%), required pleurodesis or reoperation. Ohtsuka et al. compared two groups of patients having the intersegmental plane divided by electrocautery alone and electrocautery combined with stapling (15). The incidence of PAL was higher in the group electrocautery alone than in the group combination of electrocautery and staplers [14% (3/22) vs. 4% (1/25), P=0.025]. Thus, as mentioned by Miyasaka et al., dividing the intersegmental plane by electrocautery may result in more prolonged air leaks (4). Besides, an indirect sign suggesting that splitting the intersegmental by electrocautery is a concern, is the number of studies dealing with reinforcement of the parenchymal plane by various types of sealant and/or mesh (15-18).
Lung re-expansion
Asakura et al. compared the lung expansion depending on the method used to develop the intersegmental plane based on an ex vivo swine model (3). The authors stated that stapler interferes with the expansion of preserved lung in comparison to scissors, or to a combination of scissors for thin parenchyma and stapler for thick parenchyma, due to the visceral pleura being caught in the staple line that causes atelectasis. The sample size for each group was limited (8, 8 and 3 respectively) so that no definite conclusion can be drawn from this work. Other surgeons recommend division of the intersegmental plane by electrocautery because it allows complete expansion of the residual segments (15,19). In our series, the overall rate of incomplete re-expansion at 1 month follow-up was finally limited (2.5%) and none of the patients has to undergo specific measure, such as chest drainage or reoperation. A higher incidence of incomplete lung re-expansion was noted when upper lobe segmentectomy was performed. It has been suggested that small alveolopleural fistulae may produce a low flow air leakage and thus, a neutral air space, that usually reabsorbs with gradual expansion. After lower lobe segmentectomy, those alveolopleural fistulae might be immediately covered by surrounding parenchyma, whereas, after upper lobe segmentectomy, the remaining raw lung surface remain exposed (20).
In addition our results for thoracoscopic segmentectomies compared favorably to our own institutional data on segmentectomies performed by thoracotomy where both the mean drainage duration and mean hospital stay were longer (11).
Other issues related with stapling
Stapling the intersegmental plane—despite advantages in terms of safety—is not yet the ideal method. Its handling is not that easy, especially in patients with narrow or small chest cavity. Loading thick tissues can be tedious, as their opening is still limited, and disruption of the staple line can occur (21), an adverse event that we faced four times in our series. The consequences were minimal, but led to troublesome blood loss and required hand suturing. This pitfall has been dramatically reduced with the availability of larger staples. Cost is another issue. Stapling costs more than other methods as it can require using many reloads, up to 5 in the series of Watanabe et al. (22)
Another theoretical concern is the non-preservation and/or compression of the intersegmental vein, which, according to Asakura et al., could impair gas exchanges in the preserved segment (3). To our knowledge, this has not been demonstrated. Nakano et al. have suspected that some so-called stump consolidations, i.e., partial atelectasis, observed several months after thoracoscopic segmentectomies—10% in their series of 70 cases—could be related with misidentification of the intersegmental plane (19). Whether this complication is due to the technique for identification or to the stapling method, is not clear.
In conclusion, stapling the intersegmental plane can have some drawbacks (cost, difficult handling in some patients, parenchyma plication in the preserved segments), but clinical consequences are very limited. There are few prolonged air leaks and few incomplete pulmonary re-expansions, none of them leading to chest drainage or reoperation. Stapling seems to be an acceptable compromise in terms of safety.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Gossot work as consultant for instrumentation manufacturer (Delacroix Chevalier Company). The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Institutional Review Board N° 2012-10.
References
- Whitson BA, Groth SS, Andrade RS, et al. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg 2011;92:1943-50. [Crossref] [PubMed]
- Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol 2014;9:383-9. [Crossref] [PubMed]
- Asakura K, Izumi Y, Kohno M, et al. Effect of cutting technique at the intersegmental plane during segmentectomy on expansion of the preserved segment: comparison between staplers and scissors in ex vivo pig lung. Eur J Cardiothorac Surg 2011;40:e34-8. [Crossref] [PubMed]
- Miyasaka Y, Oh S, Takahashi N, et al. Postoperative complications and respiratory function following segmentectomy of the lung - comparison of the methods of making an inter-segmental plane. Interact Cardiovasc Thorac Surg 2011;12:426-9. [Crossref] [PubMed]
- Gossot D, Ramos R, Brian E, et al. A totally thoracoscopic approach for pulmonary anatomic segmentectomies. Interact Cardiovasc Thorac Surg 2011;12:529-32. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated Anatomical Segmentectomy for Lung Cancer. Tokyo: Springer, 2011:1-248.
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg 2011;39:989-94. [Crossref] [PubMed]
- Yamashita S, Chujo M, Kawano Y, et al. Clinical impact of segmentectomy compared with lobectomy under complete video-assisted thoracic surgery in the treatment of stage I non-small cell lung cancer. J Surg Res 2011;166:46-51. [Crossref] [PubMed]
- Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg 2014;3:176-82. [PubMed]
- Bagrodia N, Cassel S, Liao J, et al. Segmental resection for the treatment of congenital pulmonary malformations. J Pediatr Surg 2014;49:905-9. [Crossref] [PubMed]
- Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg 2013;17:838-44. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: a new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Iwata H, Shirahashi K, Mizuno Y, et al. Surgical technique of lung segmental resection with two intersegmental planes. Interact Cardiovasc Thorac Surg 2013;16:423-5. [Crossref] [PubMed]
- Okada M, Tsutani Y, Ikeda T, et al. Radical hybrid video-assisted thoracic segmentectomy: long-term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012;14:5-11. [Crossref] [PubMed]
- Ohtsuka T, Goto T, Anraku M, et al. Dissection of lung parenchyma using electrocautery is a safe and acceptable method for anatomical sublobar resection. J Cardiothorac Surg 2012;7:42. [Crossref] [PubMed]
- Nomori H. Anatomical segmentectomy for clinical stage IA non-small cell lung cancer. Nihon Geka Gakkai Zasshi 2011;112:264-6. [PubMed]
- Yoshimoto K, Nomori H, Mori T, et al. Comparison of postoperative pulmonary function and air leakage between pleural closure vs. mesh-cover for intersegmental plane in segmentectomy. J Cardiothorac Surg 2011;6:61. [Crossref] [PubMed]
- Matsumura Y, Okada Y, Shimada K, et al. New surgical technique of pulmonary segmentectomy by ultrasonic scalpel and absorbable sealing materials. Kyobu Geka 2004;57:31-7. [PubMed]
- Nakano T, Endo S, Mitsuda S, et al. Stump consolidation after video-assisted thoracoscopic segmentectomy. Kyobu Geka 2011;64:792-5. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Gossot D, Merlusca G, Tudor A, et al. Pitfalls related to the use of endostaplers during video-assisted thoracic surgery. Surg Endosc 2009;23:189-92. [Crossref] [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80; discussion 780. [Crossref] [PubMed]


