Usefulness of conventional transbronchial needle aspiration in the diagnosis, staging and molecular characterization of pulmonary neoplasias by thin-prep based cytology: experience of a single oncological institute
Introduction
Conventional transbronchial needle aspiration (c-TBNA) has contributed to implement the bronchoscopic examination, allowing to sample lesions located even outside the tracheo-bronchial tree and in the hilo-mediastinal district. In addition to the original diagnostic value, the flexible bronchoscopy has also allowed the staging of pulmonary neoplasms. Although the first description of a transbronchial sampling of a mediastinal lymph node was reported in 1949 (1), the “father” of c-TBNA should be considered Ko-Pen Wang of the Baltimora J. Hopkins University who in the 80s reported on the safety and usefulness of c-TBNA in N staging of lung tumors and in the diagnosis of hilo-mediastinal masses (2,3). However, for several years, this diagnostic approach was inconsistently applied due to the limited diagnostic yield, to the fear of complications and also to the needle costs. On the basis of available evidences, the c-TBNA should be considered safer than other bronchoscopic sampling methods, such as the transbronchial biopsy, widely used among thoracic endoscopists (4). In addition, a positive cost-effectiveness of c-TBNA has been described with comparison to the traditional surgical lung neoplasm staging techniques [mediastinoscopy, mediastinotomy, video-assisted thoracoscopic surgery (VATS)] mainly if the c-TBNA is supported by the presence of a pathologist performing a “rapid on site examination” (ROSE) (5,6). Still in the last 15 years, the endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has been developed, based on a echografic guide situated on the bronchoscope, allowing both the needle visualization while crossing the bronchial wall and sampling of the mediastinal structure(s). The findings with the echoendoscope show sensibility of 94–95.7% independently from the size and location of the sampled lymph nodes (7-9). Thus, the aim of this study was to assess and discuss if the c-TBNA, associated to thin-prep based cytology, still maintains an important role in the diagnosis and in the staging of advanced lung neoplasias, on the basis of the findings of a single oncological institute.
Methods
All cases which performed the c-TBNA during the period 2005–2015 for suspicious or advanced lung neoplasia on the basis of computed tomography (CT), at times associated to positron emission tomography-CT (CT-PET), have been sorted out from the files of the bronchoscopic archives at the Thoracic endoscopy of the Thoracic Surgery of the Regina Elena National Cancer Institute, Rome, Italy. Data from 273 consecutive patients (205 males and 68 females) were collected. The demographical information and both the bronchoscopic and pathological reports have been used to establish a complex database (DB). Different additional cyto-histological approaches including bronchial brushing, bronchial washing and biopsy have been applied in association to c-TBNA in 47 cases; transbronchial biopsy has been performed subsequently in dubious cases, sometimes together. The International Association for the Study of Lung Cancer (IASLC) lymph node map was used to nominate the Lymph node stations (10). Molecular analyses were performed on the samples with “adenocarcinoma” and “probably adenocarcinoma” diagnosis. An informed consent has been signed by patients to the bronchoscopic procedure. The IRCCS Lazio Central Ethics Committee reviewed and approved the study (R.S. 833/16). Individual consent for the study was waived because the patients remained anonymous.
Bronchoscopy procedure
In the first period [2005–2011], the bronchoscopic procedure has been performed in local anaesthesia of the upper respiratory tract and light sedation with 3 mg midazolam. In the second period [2012–2015], the procedure was associated to deep sedation with a small bolus injection of anesthetic propofol and fentanyl in bolus or remifentanil in continuous infusion. As a routine procedure the c-TBNA was performed in a day hospital setting. A flexible videobronchoscopic (Olympus BFT160) was applied per os. Vital parameters were controlled and O2 was administered by a nasal cannula in order to maintain a 90% oxygen saturation. An accurate preliminary workup was performed by CT with contrast to identify the c-TBNA target and its relation to the tracheobronchial tree, and to apply correctly the needle. The c-TBNA was performed on hilar and mediastinal lymph nodes enlarged (short axis >2 cm) according to CT, suspicious for neoplasia. Two different fine needles of 21 Gauge (G) have been used: EXCELON 6410, Boston Scientific, and WANG MW-121, Conmed. Different passages were performed: at the beginning we performed one passage, between 2007–2011 at least two passages and in the period 2012–2015 we performed three or more passages. A single bronchoscopist (MF) with a longstanding expertise performed the c-TBNA procedure before any other bronchoscopic approach. In the last period, starting from March 2014 to December 2015, in 23 cases a dedicated pathologist (FM) assisted to the procedure in order to verify the adequacy of the material provided (ROSE). To be considered adequate (i.e., centred in a lymph node), the material should have been containing at least a few lymphocytes.
Cytological procedure
Since 2005, c-TBNA specimens have been treated with ThinPrep (Cytyc, Marlborough, MA, USA) process: the cytological specimen collection was placed directly into a methanol-based preservative solution (Cytyc CytoLyt); following centrifugation and discarding of the supernatant, the cell pellet was resuspended and a sample transferred to a second methanol-based preservative (Cytyc Preservcyt); subsequently, the ThinPrep processor yields an alcohol-fixed slide preparation. The processor disperses cells, collects them on a polycarbonate filter, and finally transfers the cells to a glass slide, which is then immersed in 95% alcohol and then stained with Papanicolaou technique.
Rapid on site staining technique (ROSE)
A fine needle aspiration (FNA) was placed onto a holder, and then divided into two aliquots, each of which was then transferred on a slide as a smear; specimen was fixed 20 sec in 95% ethanol, then stained in Harris hematoxylin; in contrast specimen stainings last for 30–45 sec, followed by rapid rinsing in water. Later, both samples were protected by a cover slide, without mounting medium and dried while removing any eventual air bubbles; finally, slides were examined under a light microscope.
Molecular analyses
Slides derived from cytological samples processed by the monolayer ThinPrep method and stained with Papanicolaou were reviewed by two pathologists with specific expertise in cytology (FM, PV) and samples with at least 50% neoplastic cells were used for molecular analysis. The Thin Prep material stored in the vial was used for DNA isolation performed with the QIAmp DNA kit (Qiagen) according to the manufacturer’s instructions.
EGFR gene mutational analysis
In the period 2010–2012, exons 19–21 of EGFR gene and exon 2 of KRAS gene were PCR amplified by AmpliTaq Gold 360 Master Mix (Applied Biosystems, Foster City, CA, USA). All PCR products were directly sequenced with the V3.1 Big Dye Terminator Cycle Sequencing Reaction Kit (Applied Biosystems) and then run on ABI PRISM 3130 (Applied Biosystems). Clustaw2 alignment software EMBL-EBI was used to perform sequencing analysis.
Real-time PCR
In the period 2013–2015, EGFR (exon 18-19-20-21) and K-RAS (exon 2-3-4) mutation status was evaluated by a rapid and sensitive real time TaqMan assay method (EntroGen, Inc.) with EGFR/KRAS reporter FAM, quencher NFQ-MGB, control reporter VIC. Amplification and allelic discrimination were performed on an Applied Biosystems 7500 Real Time PCR system.
Fluorescence in situ hybridization (FISH) of ALK, ROS1 rearrangements
ALK rearrangements at 2p23 chromosome locus and ROS1 rearrangements at 6q22 chromosome locus were investigated by FISH. To detect ALK translocation the ALK FISH DNA Probe 2p23 Split Signal Code Y5417 was used (Dako, Milan, Italy). To detect ROS1 rearrangements ROS1 IQFISH Break-Apart Probe 6q22 Code G111601-8 was used (DAKO, Agilent Genomics). The results of the hybridization were interpreted according to the manufacturer’s instructions and current guidelines on molecular testing (11-15).
Statistical analysis
Sensitivity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of the tests were calculated according to the standard definitions as follows: sensitivity = true positives/true positives + false negatives; accuracy = true positives + true negatives/total population; PPV = true positives/true positives + false positives; NPV = true negatives/true negatives + false negatives. Data were reported as mean ± standard deviation (SD). The confidence intervals have been calculated with the following formula: (accuracy, PPV, NPV) ±1.96× standard error (SE). The probability area between the points of abscissa −1.96 and +1.96 is 0.95. A zi point in a standard normal distribution has the probability of 95% to fall within the range considered, and 5% of falling outside. Microsoft Excel was used for data entry and statistical analysis.
Results
Among the 273 total cases (205 patients were males, 68 were females, mean age 65±11 y) with suspected lung cancer and involvement of mediastinal lymph nodes, 158 (58%) c-TBNA samples have been considered adequate for diagnostic purposes. In the remaining 115 (42%) cases a diagnosis was not possible because the samples were considered inadequate (i.e., containing only bronchial normal epithelial cells and/or red cells) (Table 1). Among 158 adequate specimens, 112 were frankly neoplastic or with atypical cells, 46 were negative for malignant/atypical cells, four out of the 46 negative cases proved to be positives for malignancy on subsequent biopsy (false negative). The most frequent histological type was adenocarcinoma (32%), followed by small cell lung carcinoma (SCLC) (16%), non small cell lung carcinoma (NSCLC), NOS (9%) and squamous cell carcinoma (SQCC) (7%); 9 patients had atypical cells, (epithelial and/or lymphoid) and 3 patients had metastatic tumours (2 from breast cancer, 1 from melanoma) (Table 2).

Full table

Full table
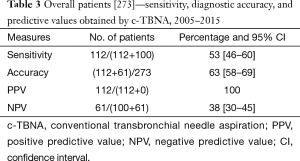
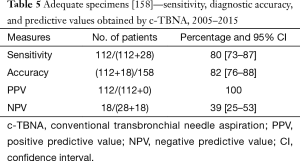
The number of the procedures performed varied along the years; in the first three years, only 43 procedures were performed, on the whole; therefore we report overall data for years 2005–2007. A relative decrease in the years 2009–2011 was related to the contemporary initial activity of the endoscopic ultrasound trans-esophageal FNA (EUS-FNA) on the posterior mediastinal lymph node stations, performed at the Gastrointestinal Endoscopy Department. A strong increase in adequacy has to be noticed in the period 2013–2015. Therefore we considered the sensibility, accuracy and PPV and NPV on the overall period (Table 3) and in the two periods separately (2005–2011 and 2012–2015) (Table 4). During the overall period, sensibility and accuracy values were respectively of 53% and 63% (Table 5). In the first period [2005–2011] sensibility and accuracy were 41% and 53% respectively; in the second period [2012–2015] sensibility and accuracy raised to 60% and 68% (Table 6). The PPV always reach 100%; NPV, rather low at the beginning (31%), in an evaluation of the two periods together or in the second period reached the value of 38%. Considering only the 158 adequate specimens, sensibility and accuracy during the overall period were respectively of 80% and 82%; in the first period [2005–2011] sensibility and accuracy reached 68% and 72%; finally, in the second period sensibility reached 86% and accuracy 89% (Table 6).
Full table

Full table
Full table

Full table
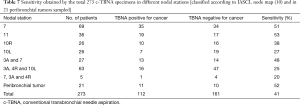
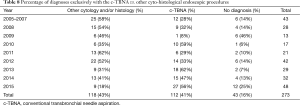
In the overall period 2005–2015, the lymph node stations by frequency have been the subcarinal (station 7: 69 cases), the superior mediastinal (3A, 4R) and together the hilar (total: 63 cases), hilar-lobar (station 11: 36 cases), superior mediastinal and subcarinal together (3A and 7, 27 cases) right and left hilar principal (10R 26 cases and 10L 26 cases); 21 peribronchial tumors were also investigated by c-TBNA, 11 out of 21 samples being positive (Table 7). The higher sensitivity was achieved for the station 11, followed by the subcarinal station 7 and by the station 7 together with the 3A station (Table 7). Among the 112 cases adequate and positive for neoplasia, 65 cases had the diagnosis exclusively with the c-TBNA, in 47 cases other cyto-histological endoscopic procedures were associated. Therefore the c-TBNA alone was diagnostic in variable percentage of cases, with a peak of 59% in 2010 and 62% in 2015; during the overall period, 43 cases were lost to follow up and it was not possible to establish the diagnosis. The results derived from c-TBNA in comparison with the final diagnosis were shown in Table 8.
Full table
Full table
Molecular genetic determinations: in the period 2010–2015 in 30 out of 50 adenocarcinoma/likely adenocarcinoma cases a molecular investigation has been successful, in the first two years by investigating only the EGFR gene. Later, by sequential analyses, other relevant genes were also investigated: K-RAS, ALK and ROS1. On the whole, 48 single and/or sequential evaluation of multiple genes were performed. Table 9 shows the percentages of the molecular determinations compared to the number of patients affected by adenocarcinoma. In the overall period an average of 60% of cases were tested by molecular biology, with a maximum of 80% in 2013.

Full table
Discussion
As clearly demonstrated from the literature, c-TBNA results are largely influenced by several parameters: lymph node target size (>2 cm), passage number, the improvement in the endoscopist’s skill, the needle size and the presence of a pathologist on site (16,17).
We collected and analyzed the c-TBNA data from a series of 273 patients with suspect or advanced lung cancer. The patient series has been analysed in two periods, 2005–2011 and 2012–2015. The size of the lymph nodes varied between 2 and 6 cm, the c-TBNA finding the prevalent indication when the suspected lymph nodes had a diameter >2 cm. The passage number varied along the years: as also shown in the literature (17), being single in the first 2 years [2005–2006], double in the period 2007–2011 and at least triple (until a maximum of five passages) in the period 2012–2015. Accordingly, in the first 2 years the sensibility was of 41%, with 57 cases out of 122 inadequate, and accuracy was of 53%. In the second period every single lymph node station has been stinged at least 3 times until to a maximum of 5 passages, and the sensibility raised to 60% with a parallel decrease of inadequate samples (48 out of 151 samples) and the accuracy reached the 68%. The hilar stations (N1) have been always reached with high sensibility and accuracy, whereas the mediastinal stations (N2) at the beginning were often non diagnostic or no accuracy was reached; in the second period 2012–2015, however, the sampling of the N2 stations became very often diagnostic. The best results were obtained on the lymph node stations 11, 7 e 3A, in relation with the best endobronchial landmarks available for the optimal aspiration site. A crucial point is the endoscopist’s experience: the technique in itself is simple and it is possible to learn “by the book” and with exercise on inert models when no formal training is possible (18). Literature data point on the necessity of at least 50 procedures, assisted by an experienced endoscopist (19-24). In our study the percentage of adequate cases rose from 52% in the first period to 62% in the second period. Similar results had been reported by other studies (25). Needle size doesn’t appear to be relevant in order to ensure the diagnostic yield: several studies demonstrated that hilar and mediastinal lymph nodes with relevant neoplastic involvement may be sampled both with needle for cytology (21 G) and with needle for histology (19 G). For suspicious lymphoma or granulomatous disease the needle providing the best diagnostic yield (19 G) should be used after adequate training and experience of the endoscopist with fine needle 20–21 G (16).
According to the Guidelines of the American College of Chest Physicians (26), the presence on site of a pathologist is not recommended: however, in our experience, the pathologist presence improve the diagnostic yield and decreases the costs, either for the material reduction either by avoiding further investigations. In our study, in fact, the adequacy reached the 71% in the last year, when we adopted the ROSE. The results obtained with regard to sensibility and accuracy were comparable to those reported with the help of echography (EBUS-TBNA) (26-28).
In our experience we didn’t observed complications, such as described in the literature, neither anaesthesiologic problems. Actually the rare complications associated to c-TBNA include fever, slight bleeding and bacterial pericarditis. Moreover mediastinitis and pneumothorax have been described, usually with spontaneous and favorable resolution (29,30).
The recent revision of adenocarcinoma classification in different subtypes proved to be clinically relevant; different genic profiles have been associated to the histologic subtypes, bearing prognostic and predictive value (31-34). Molecular testing of lung adenocarcinoma for the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) is now considered standard of care and part of the diagnostic algorithm together with a search for other “driver mutations” in oncogenes such as KRAS, ROS1 (11). EGFR mutations are present in approximately 15% of primary lung adenocarcinomas and are mutually exclusive of KRAS and ALK mutations (35). Rearrangements of ROS1 appear mutually exclusive of other known oncogenic drivers (https://www.mycancergenome.org/content/disease/lung-cancer/ros1/67/).
At the moment predictive molecular tests are usually performed on the primary tumor (T). Although the first studies correlating genetic alterations in metastatic lymph nodes have been deluding (36-38), other reports demonstrated that primary tumor mutational status not always correlate with the metastatic lymph node genetic status (39). Chen et al. showed that the therapeutic response to tyrosine kinase inhibitor (TKI) acting on EGFR is more pronounced in patients with mutated tumor in the metastatic foci (40): therefore the mutational status of the N component could be more predictive of therapy response than that of the primary tumor (41).
Although in the literature it is not possible to compare the efficacy of c-TBNA vs. EBUS-TBNA in molecular studies, it has been shown that the number of passages improves the diagnostic yield (42). In our study the molecular diagnostic yield increased in parallel with the c-TBNA sensibility and accuracy, the samples being sufficient to provide molecular data in 60% of adenocarcinoma samples, with a relevant increase from 2010 (33% of adequate cases) to 2015 (67% of cases effective in molecular characterization), with a maximum of 80% in 2013. The availability of the thin-prep technique offered a consistent improvement of diagnosis and provided material for molecular analyses, comparable with the results obtained by histological samples/biopsies (43-45).
Finally in our study the c-TBNA was the first and unique diagnostic procedure in 41% of total cases (65/158), avoiding major, invasive and expensive diagnostic procedures. Associated to other diagnostic procedures (brushing, BAL, biopsy) the c-TBNA proved to be diagnostic in 71% of cases (112/158).
Conclusions
Our study show demonstrated that the c-TBNA today still represents an efficient method for diagnosis and staging of advanced lung tumours (46,47). The diffusion of EBUS certainly improved the sensibility, however, whenever the more advanced technologies are not available, the c-TBNA represents a milestone in diagnostics (48). Moreover, the initial phase of EBUS setting and the needles are very expensive. Therefore only referral centers, at the moment, can afford these expenses. The c-TBNA could be performed also during the first diagnostic bronchoscopy in case of suspicious lung tumour and it could represent the first and unique diagnostic procedure, if positive. More complex or more invasive procedures are indicated whenever the c-TBNA doesn’t provide the diagnosis. EBUS is indicated as first diagnostic procedure in case of lymph node diameter < to 2 cm or in not easily reachable sites, such as stations 2R, 2L, 4L or lower subcarinal. Therefore c-TBNA is a complex but affordable procedure to be performed, with low costs, even in peripheral but well trained centers of thoracic expertise.
Acknowledgements
The authors thank Dr. Diana Giannarelli, M.Sc, Biostatistic Unit, Regina Elena National Cancer Institute, Rome, Italy, for the Statistical consultation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the IRCCS Lazio Central Ethics Committee (No. R.S. 833/16). A written informed consent to the procedure was obtained from all patients.
References
- Schieppati E. Mediastinal puncture thru the tracheal carina. Rev Asoc Med Argent 1949;63:497-9. [PubMed]
- Wang KP, Brower R, Haponik EF, et al. Flexible transbronchial needle aspiration for staging of bronchogenic carcinoma. Chest 1983;84:571-6. [Crossref] [PubMed]
- Wang KP, Haponik EF, Britt EJ, et al. Transbronchial needle aspiration of peripheral pulmonary nodules. Chest 1984;86:819-23. [Crossref] [PubMed]
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [Crossref] [PubMed]
- Bruno P, Ricci A, Esposito MC, et al. Efficacy and cost effectiveness of rapid on site examination (ROSE) in management of patients with mediastinal lymphadenopathies. Eur Rev Med Pharmacol Sci 2013;17:1517-22. [PubMed]
- Sindhwani G, Rawat J, Chandra S, et al. Transbronchial needle aspiration with rapid on-site evaluation: a prospective study on efficacy, feasibility and cost effectiveness. Indian J Chest Dis Allied Sci 2013;55:141-4. [PubMed]
- Arslan Z, Ilgazli A, Bakir M, et al. Conventional vs. endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymphadenopathies. Tuberk Toraks 2011;59:153-7. [Crossref] [PubMed]
- Zhu T, Zhang X, Xu J, et al. Endobronchial ultrasound guided-transbronchial needle aspiration vs. conventional transbronchial needle aspiration in the diagnosis of mediastinal masses: A meta-analysis. Mol Clin Oncol 2014;2:151-5. [PubMed]
- Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828-60. [Crossref] [PubMed]
- Alì G, Proietti A, Pelliccioni S, et al. ALK rearrangement in a large series of consecutive non-small cell lung cancers: comparison between a new immunohistochemical approach and fluorescence in situ hybridization for the screening of patients eligible for crizotinib treatment. Arch Pathol Lab Med 2014;138:1449-58. [Crossref] [PubMed]
- Tsao MS, Hirsch FR, Yatabe Y. editors. IASCL Atlas of ALK testing in lung cancer. Aurora: IASLC Press, 2013.
- Shan L, Lian F, Guo L, et al. Detection of ROS1 gene rearrangement in lung adenocarcinoma: comparison of IHC, FISH and real-time RT-PCR. PLoS One 2015;10:e0120422. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Bonifazi M, Zuccatosta L, Trisolini R, et al. Transbronchial needle aspiration: a systematic review on predictors of a successful aspirate. Respiration 2013;86:123-34. [Crossref] [PubMed]
- Chin R Jr, McCain TW, Lucia MA, et al. Transbronchial needle aspiration in diagnosing and staging lung cancer: how many aspirates are needed? Am J Respir Crit Care Med 2002;166:377-81. [Crossref] [PubMed]
- Kupeli E, Memis L, Ozdemirel TS, et al. Transbronchial needle aspiration "by the books". Ann Thorac Med 2011;6:85-90. [Crossref] [PubMed]
- Rodríguez de Castro F, Díaz López F, Serdà GJ, et al. Relevance of training in transbronchial fine-needle aspiration technique. Chest 1997;111:103-5. [Crossref] [PubMed]
- Bellinger CR, Chatterjee AB, Adair N, et al. Training in and experience with endobronchial ultrasound. Respiration 2014;88:478-83. [Crossref] [PubMed]
- Tutar N, Büyükoğlan H, Yılmaz I, et al. Learning curve of conventional transbronchial needle aspiration. Clin Respir J 2014;8:79-85. [Crossref] [PubMed]
- Küpeli E, Seyfettin P, Tepeoğlu MD. Conventional transbronchial needle aspiration: From acquisition to precision. Ann Thorac Med 2015;10:50-4. [PubMed]
- Haponik EF, Cappellari JO, Chin R, et al. Education and experience improve transbronchial needle aspiration performance. Am J Respir Crit Care Med 1995;151:1998-2002. [Crossref] [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration 2010;79:482-9. [Crossref] [PubMed]
- Fuso L, Varone F, Smargiassi A, et al. Usefulness of Conventional Transbronchial Needle Aspiration for Sampling of Mediastinal Lymph Nodes in Lung Cancer. J Bronchology Interv Pulmonol 2015;22:294-9. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:202S-220S.
- Jiang J, Browning R, Lechtzin N, et al. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thorac Dis 2014;6:416-20. [PubMed]
- Bellinger CR, Chatterjee AB, Chin R Jr, et al. Conventional and endobronchial ultrasound-guided transbronchial needle aspiration: complementary procedures. South Med J 2012;105:625-9. [Crossref] [PubMed]
- Epstein SK, Winslow CJ, Brecher SM, et al. Polymicrobial bacterial pericarditis after transbronchial needle aspiration. Case report with an investigation on the risk of bacterial contamination during fiberoptic bronchoscopy. Am Rev Respir Dis 1992;146:523-5. [Crossref] [PubMed]
- Boskovic T, Stojanovic M, Stanic J, et al. Pneumothorax after transbronchial needle biopsy. J Thorac Dis 2014;6:S427-34. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. [Crossref] [PubMed]
- Sun Z, Yang P. Gene expression profiling on lung cancer outcome prediction: present clinical value and future premise. Cancer Epidemiol Biomarkers Prev 2006;15:2063-8. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edition. Lyon: IARC, 2015:412.
- Khoo C, Rogers TM, Fellowes A, et al. Molecular methods for somatic mutation testing in lung adenocarcinoma: EGFR and beyond. Transl Lung Cancer Res 2015;4:126-41. [PubMed]
- Ahrendt SA, Yang SC, Wu L, et al. Molecular assessment of lymph nodes in patients with resected stage I non-small cell lung cancer: preliminary results of a prospective study. J Thorac Cardiovasc Surg 2002;123:466-73; discussion 473-4. [Crossref] [PubMed]
- Harden SV, Tokumaru Y, Westra WH, et al. Gene promoter hypermethylation in tumors and lymph nodes of stage I lung cancer patients. Clin Cancer Res 2003;9:1370-5. [PubMed]
- Brock MV, Hooker CM, Ota-Machida E, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med 2008;358:1118-28. [Crossref] [PubMed]
- Wang F, Fang P, Hou DY, et al. Comparison of epidermal growth factor receptor mutations between primary tumors and lymph nodes in non-small cell lung cancer: a review and meta-analysis of published data. Asian Pac J Cancer Prev 2014;15:4493-7. [Crossref] [PubMed]
- Chen ZY, Zhong WZ, Zhang XC, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 2012;17:978-85. [Crossref] [PubMed]
- Nana-Sinkam SP, Powell CA. Molecular biology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e30S-9S.
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [PubMed]
- Petriella D, Galetta D, Rubini V, et al. Molecular profiling of thin-prep FNA samples in assisting clinical management of non-small-cell lung cancer. Mol Biotechnol 2013;54:913-9. [Crossref] [PubMed]
- Zhang Z, Yuan P, Guo H, et al. Assessment of Hormone Receptor and Human Epidermal Growth Factor Receptor 2 Status in Breast Carcinoma Using Thin-Prep Cytology Fine Needle Aspiration Cytology FISH Experience From China. Medicine (Baltimore) 2015;94:e981. [Crossref] [PubMed]
- Tripathy K, Misra A, Ghosh JK. Efficacy of liquid-based cytology versus conventional smears in FNA samples. J Cytol 2015;32:17-20. [Crossref] [PubMed]
- Trisolini R, Gasparini S. Is it time for conventional TBNA to die? J Bronchology Interv Pulmonol 2013;20:368-9. [Crossref] [PubMed]
- Yang H, Zhang Y, Wang KP, et al. Transbronchial needle aspiration: development history, current status and future perspective. J Thorac Dis 2015;7:S279-86. [PubMed]
- Trisolini R, Patelli M, Gasparini S. While waiting to buy a ferrari, do not leave your current car in the garage! Respiration 2010;79:452-3. [Crossref] [PubMed]


